Description
3M 1688 - DRESSING, TEGADERM IV, ADVANCED, 4"X4.75", 50/BX
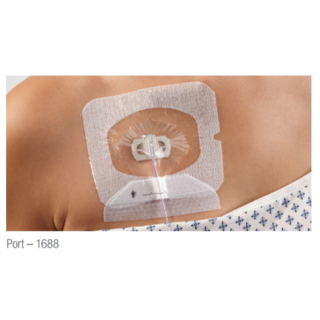
3M Tegaderm I.V. Advanced Securement Dressing 1688, 4 in. x 4.75 in. (10 cm x 12 cm)
Designed for Securement & Comfort
For Pediatrics
3M Tegaderm I.V. Advanced Securement Dressings are comprehensively designed to deliver exceptional patient care through advanced catheter securement, superior wear time and gentle application and removal. Two new specialty sized dressings help pediatric clinicians care for their diverse and critically important patient population.
- A line of I.V. dressings which optimises all characteristics to provide advanced I.V. and central line catheter securement
- A cost-effective I.V. and central line catheter securement solution
- Innovative adhesives balance securement and gentle removal
- Easy to apply and remove
- Provides up to 7 days of wear time for PIVs or CVCs
Suggested Applications
- Short peripheral and midline venous catheters
- Central venous catheters (including subclavian, jugular, femoral and PICCs)
- Arterial catheters
- Tunneled central vascular devices
- Enteral feeding tubes
- Dialysis catheters
- Adult and pediatric venous catheters
- Pulmonary artery catheters

Helping You Deliver Exceptional Patient Care
Every day, we''re inspired by all you do. Were committed to helping you with people, products and ideas that simplify and improve the standard of I.V. site care. Thats why we leveraged our customer insights and proven leadership to help you provide the comfort and protection your patients deserve.
Provides secure hold
Reinforced border, deep notch, and tape strips work together to enlist the entire dressing in securement. Innovative adhesives hold strongly, manage moisture, and release easily. Designed to maintain securement by preventing edge lift, flexing with patient movement, and managing moisture.
Easy to apply and remove
Designed to promote consistent application. Frame delivery makes placement accurate and easy.
Excellent value
Can potentially reduce the number of dressing changes and restarts. May be worn for up to 7 days on a PIV catheter. Provides up to 7 days of wear time for CVCs**
Supports infection prevention goals
A waterproof film coating on all pieces provides a barrier to external contaminants including liquids, bacteria and viruses*. Allows continuous monitoring.
Improves compliance to best practices and protocols
3M Tegaderm I.V. Advanced Securement Dressings meet the industry definitions of a catheter securement device and support the goals of other professional guidelines and facility protocols for improving patient outcomes.
FDA Classification FDA 21 CFR880.5210: An intravascular catheter securement device is a device with an adhesive backing that is placed over a needle or catheter and is used to keep the hub of the needle or the catheter flat and securely anchored to the skin.
Centers for Disease Control 2011 HICPAC Guidelines for the Prevention of Intravascular Catheter-Related Infections (Category II): Use a sutureless securement device to reduce the risk of infection for intravascular catheters.
Infusion Nursing Standards of Practice (2011), Standard 36: Vascular Access Device Stabilization (VAD) Practice Criteria A: The use of a catheter stabilization device should be considered the preferred alternative to tape or sutures when feasible. Both the CDC and the INS recommend the use of transparent film dressings to protect I.V. sites because they allow continuous site observation. Tegaderm I.V. Advanced Securement Dressings can be safely worn for up to seven days - until the dressing is soiled or no longer intact - or for the length of facility protocol.

Supports infection prevention goals
The transparent film allows for effective oxygen-vapor exchange while helping protect against external contaminants, including those most commonly associated with catheter-related bloodstream infections.

Patterned Adhesive System
A breathable patterned adhesive system added to the border and tape strips, designed to maximize securement, breathability and wear time.

Conforming Edge Border
A new border technology designed to increase conformability and reduce edge-lift.


Out-Performs Against Leading Transparent Film Dressing
When securing a straight PIV catheter, 3M Tegaderm I.V. Advanced Securement Dressing 1683 plus tape strips withstood three times the pull force after 48 hours of the leading flat film dressing plus tape strip (see Figure 1).
Innovative adhesives provide excellent hold for the length of your facilitys protocol. The patterned adhesive secures and protects the catheter and channels moisture away from the skin to help reduce the risk of maceration. A second, complementary adhesive around the reinforced border further enhances securement.

Can Provide Significant Cost Savings
Tegaderm I.V. Advanced Securement Dressings can help facilities manage I.V. site care costs by potentially reducing the number of dressing changes and catheter restarts. The transparent film allows for continuous monitoring of the insertion site. The dressing may be worn for the life of a PIV catheter and provides up to 7 days of wear time for CVCs.** A waterproof film coating on all pieces resists soiling and provides a barrier to external contaminants including liquids, bacteria and viruses.
Over the course of a year, facilities can realize significant savings using Tegaderm I.V. Advanced Securement Dressings in place of tape plus transparent film dressing as part of their standard of care.
Application & Removal Guide

Specifications

Disposal Considerations
Dispose of waste product in a permitted industrial waste facility. As a disposal alternative, incinerate in a permitted waste incineration facility. Proper destruction may require the use of additional fuel during incineration processes. Empty and clean product containers may be disposed as non-hazardous waste. Consult your specific regulations and service providers to determine available options and requirements. Dispose of waste product in a permitted industrial waste facility. As a disposal alternative, incinerate in a permitted waste incineration facility. Proper destruction may require the use of additional fuel during incineration processes. If no other disposal options are available, waste product may be placed in a landfill properly designed for industrial waste. Packaging (that may or may not contain any residual substance) may be lawfully disposed of by householders or other consumers through public or commercial waste collection services.