Description
3M 9525HP - Dressing Tegaderm HP IV 2-1/2x2-3/4" Flm Transparent Adh 400/Ca

3M Tegaderm IV Securement Dressing Designed for the BD Nexiva Closed IV Catheter System 9525HP, 5½ in. x 6½ in. (14 cm x 16,5 cm), 100 Dressings/Box, 4 Boxes/Case
Background
Numerous scientific investigations conducted since the 1970s have examined the role of dressings for I.V. site and wound care. This research demonstrated the importance of the dressing for I.V. protection and enhanced wound healing, and stimulated the search for dressing materials with properties similar to healthy, intact skin. Semi-permeable transparent film dressings were designed to provide this improved environment. They prevent the passage of liquids, bacteria and viruses* to the I.V. site or wound, while allowing moisture vapor and gas exchange through the dressing. These properties, together with transparency and conformability, make transparent film dressings an excellent complement to I.V. catheter care and wound management protocols. 3M Tegaderm Transparent Film Dressing is the preferred transparent dressing worldwide.
The clear choice for easy application and extended wear time
3M Tegaderm HP Transparent Film Dressings consist of a thin film backing with a hypoallergenic, latex-free adhesive that gently, yet securely, adheres to skin. Tegaderm dressings are breathable, sterile, transparent and waterproof, and provide a barrier to external contaminants. Tegaderm HP Film has a special adhesive for greater holding power in the presence of moisture. Specially designed Tegaderm dressings, with unique shapes and securing tapes provide solutions for difficult to dress wounds and I.V. catheters.
- Designed specifically to reduce catheter dislodgement
- Judged by clinicians as an excellent alternative in decreasing catheter movement, dislodgements and fall-outs compared to current stabilization techniques
- Better at conforming around the BD NexivaTM Catheter System as compared to standard IV dressings
- Perceived by clinicians to wear longer than current standard film dressings
- Transparent, breathable and impermeable to liquids, viruses and bacteria.
Product Features and Benefits
Versatile - One Product to Satisfy Many Clinical Situations
Tegaderm dressings can be used to protect I.V. sites, enhance wound healing, prevent skin breakdown, and protect clean, closed surgical incisions. Tegaderm dressings are available in many sizes, shapes and application styles to meet a wide variety of needs. The frame allows the dressings to be tailored for special applications, when desired. Application systems of most other transparent dressings do not allow for this customization.
Easy to Apply - Unique Frame Delivery System
Application of Tegaderm dressing is intuitive and quick, making it easy to remember and easy to teach. It is especially convenient for patient self-care. Tegaderm dressing minimizes application time and saves dressing waste and costs. The frame delivery system provides maximum control of the thin film for rapid application of even the largest dressings. The unique "picture-frame" allows precise and secure placement of the dressing every time. If the adhesive surface accidentally touches itself, the dressing can be separated and applied, eliminating wasted dressings. Tegaderm dressing is also available in a first-aid style delivery system for the health care professional who prefers this application method.
Gentle Adhesive - Just the Right Balance in Adhesive Strength
Tegaderm dressings are made with a hypoallergenic, latex-free adhesive that is gentle to the skin, yet securely holds catheters and other devices in place. Tegaderm dressing provides good initial adhesion without building to excessive levels over time. Even for dressings left in place for extended periods, the risk of patient discomfort and skin trauma is minimal when the dressing is properly removed.
Breathable - Lets Oxygen In and Moisture Vapor Out
The breathability of Tegaderm dressings allows moisture vapor and gas exchange, which is essential to maintain normal skin function under the dressing. Patients can wear Tegaderm dressings for extended periods of time, with minimal risk of skin irritation or maceration, and without excessive proliferation of skin flora.
Waterproof, Sterile Barrier - Impervious to Liquids, Bacteria and Viruses
Tegaderm dressing acts as a barrier to protect the I.V. site or wound from external contaminants such as bacteria, viruses, blood and body fluids. Because Tegaderm dressings are waterproof, patients may bathe, shower or swim, if the dressing is completely sealed around the catheter or wound. Tegaderm dressing is sterile and remains so as long as the outer package is intact. Do not resterilize by gamma, steam, or E-beam.
Conformable - Flexes with Skin for Greater Patient Comfort
3M Tegaderm Transparent Film Dressing conforms to body contours, stretches easily, and prevents stress on the skin when the patient moves. It protects skin and bony prominences from abrasion, and allows the patient to move easily. Tegaderm dressing is comfortable to wear and presents a flat profile. The special shapes of Tegaderm dressings conform easily on difficult-to-dress areas, such as jugular and PICC insertion sites, and sacral wounds.
Enhanced Wound Healing - Improved Outcomes and Patient Comfort
Tegaderm dressing seals in natural wound fluid to maintain a moist environment, which has been shown to enhance the healing process. It prevents scab formation and dehydration of the wound bed, which can occur with conventional dry dressings. Tegaderm dressing provides a wound environment that allows epithelial cells to migrate easily across the wound surface, reducing pain caused by wound dehydration, and increases patient comfort.
Transparent - Allows Wounds and I.V. Sites to Be Easily Monitored
The transparency of Tegaderm dressing provides complete visibility of the site during application. It also allows continuous monitoring of the I.V. site or wound without disturbing or removing the dressing. This visualization eliminates unnecessary dressing changes and saves nursing time.
Fewer Dressing Changes - Increased Patient Comfort
With Tegaderm dressing, fewer dressing changes mean improved patient comfort and the risk of skin trauma from repeated adhesive removal is reduced. For I.V. care, fewer dressing changes result in less catheter manipulation, and reduced exposure to potential outside contaminants. For wound applications, the longer wear time of Tegaderm dressing allows the wound to remain undisturbed, thereby preventing disruption of the healing process.
Affordable - can be worn longer than tape and gauze dressings
The HICPAC/CDC Guideline for the Prevention of Intravascular Catheter-Related Infection supports longer wear times for transparent dressings than tape and gauze dressings for I.V. applications.13 Less frequent dressing changes save nursing time and supply costs such as preps, gloves, and dressing materials.
Clinically proven - efficacious for I.V. site and wound care
Numerous clinical trials by independent researchers support the use of Tegaderm transparent dressings for both I.V. site and wound care. Internationally recognized I.V. guidelines base site care recommendations on clinical studies using Tegaderm dressings. Most of the major I.V. studies have been conducted on high-risk patients with central catheters. The results of the largest, prospective randomized trials of central line dressings have proven that Tegaderm dressings are as safe as gauze and tape, even when worn for longer periods of time. The extended wear times of Tegaderm dressings did not increase the risk of I.V. catheter-related bacteremias.
Several studies of peripheral I.V. catheters have also been conducted. The largest of these, with 2088 catheters, demonstrated the safety and cost-effectiveness of Tegaderm dressings left in place for the duration of catheterization.
Wound studies have demonstrated the importance of transparent dressings for rapid healing, protection of the wound from external contamination, and patient comfort. Clinical dossiers, one for the use of Tegaderm dressings for I.V. Therapy and the other for Wound Care, are available from your 3M representative upon request.
Radiologically transparent
Tegaderm dressing is radiologically transparent. Removing the dressing from a patient prior to x-ray is not necessary.
Tegaderm dressings are breathable, sterile, transparent and waterproof, and provide a barrier to external contaminants.
Physical Properties/Definitions
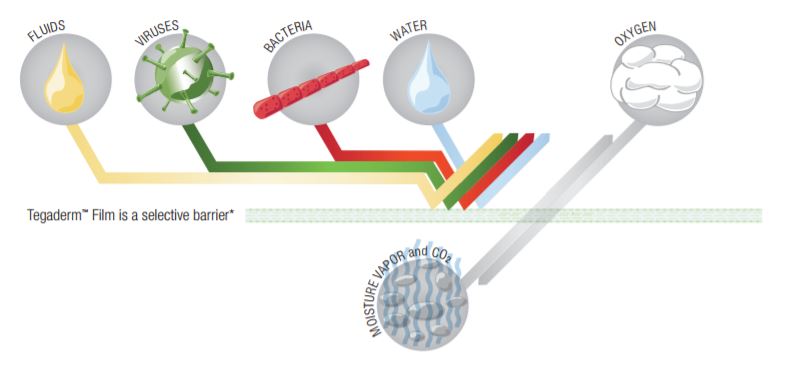
Semi-Occlusive (Semi-Permeable)
Tegaderm and Tegaderm HP Transparent Film Dressings are made of semi-permeable films. They can be thought of as selective filters-they are occlusive to liquids, bacteria, and viruses;* yet water vapor, oxygen, and carbon dioxide can easily be exchanged. Tegaderm and Tegaderm HP Film dressings are breathable. The breathability of a material is generally described in terms of oxygen and moisture vapor transmission rates (MVTR). Both rates are determined by the amount of gas that travels through the dressing in a given period of time, under specific conditions of temperature and humidity.
MVTR (Moisture Vapor Transmission Rate)
Moisture vapor transmission rate (MVTR) is the measurement of water vapor diffusion through a material. Two laboratory test methods are commonly used to measure MVTR. The results of these two tests are often used to compare transparent dressings for I.V. use. However, they do not represent real life conditions, and numerous variables can impact the results. This raises the question of whether laboratory test data for MVTR can accurately predict dressing performance in clinical practice.
The inverted beaker test produces higher numbers with greater variability. These variances are seen within samples of the same dressing, as well as among different products. This inconsistency occurs because the films can stretch and swell due to the water pressure against the test dressing, increasing the surface area measured. The MVTR values produced by the upright method are lower and more consistent among different products, and within samples of the same dressing. Because the liquid does not come in contact with the film in this test method, stretch and swell are not factors in the results.
Aside from the test method chosen, many other variables can dramatically affect moisture vapor transmission rates.
- Volume of liquid in the test beaker (generally 1050 ml)
- Type of liquid medium (water, saline)
- Concentration of substances in the liquid (salt, proteins)
- Environmental conditions (temperature, humidity)
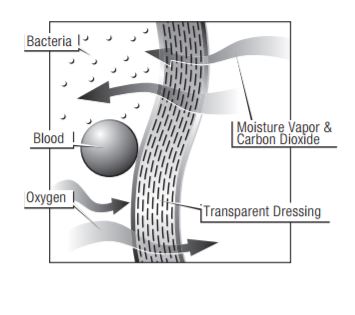
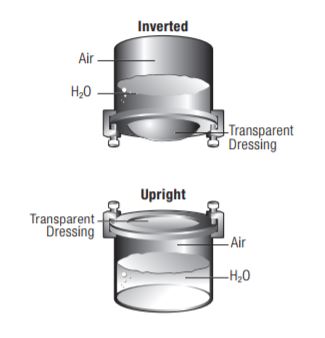
MVTR bench tests are generally performed under tightly controlled temperatures and low relative humidity. In clinical settings, where temperatures and humidity vary considerably from typical test conditions, MVTR numbers will be much different than those produced in the laboratory. For example, under conditions of high humidity, moisture vapor transmission will proceed at a much slower rate.
A third, less common method, uses computerized evaporimetry to measure moisture handling properties of transparent dressings. This instrument records actual evaporation through the film on skin, and moisture build-up underneath the dressing. When moisture vapor transmission is measured with this device, dressings with significant differences in bench MVTRs show no significant difference in actual moisture accumulation on the skin.
Suggested Applications
- To cover and protect I.V. catheter sites
- Acute and chronic wounds
- Skin protection
- Feeding tubes
- Superficial partial thickness burns
- Stage I or II pressure ulcers
- Autolytic debridement facilitated by the moist wound healing environment
- Protective eyelid coverings
- Post tattoo application and removal
Application and Removal Instructions
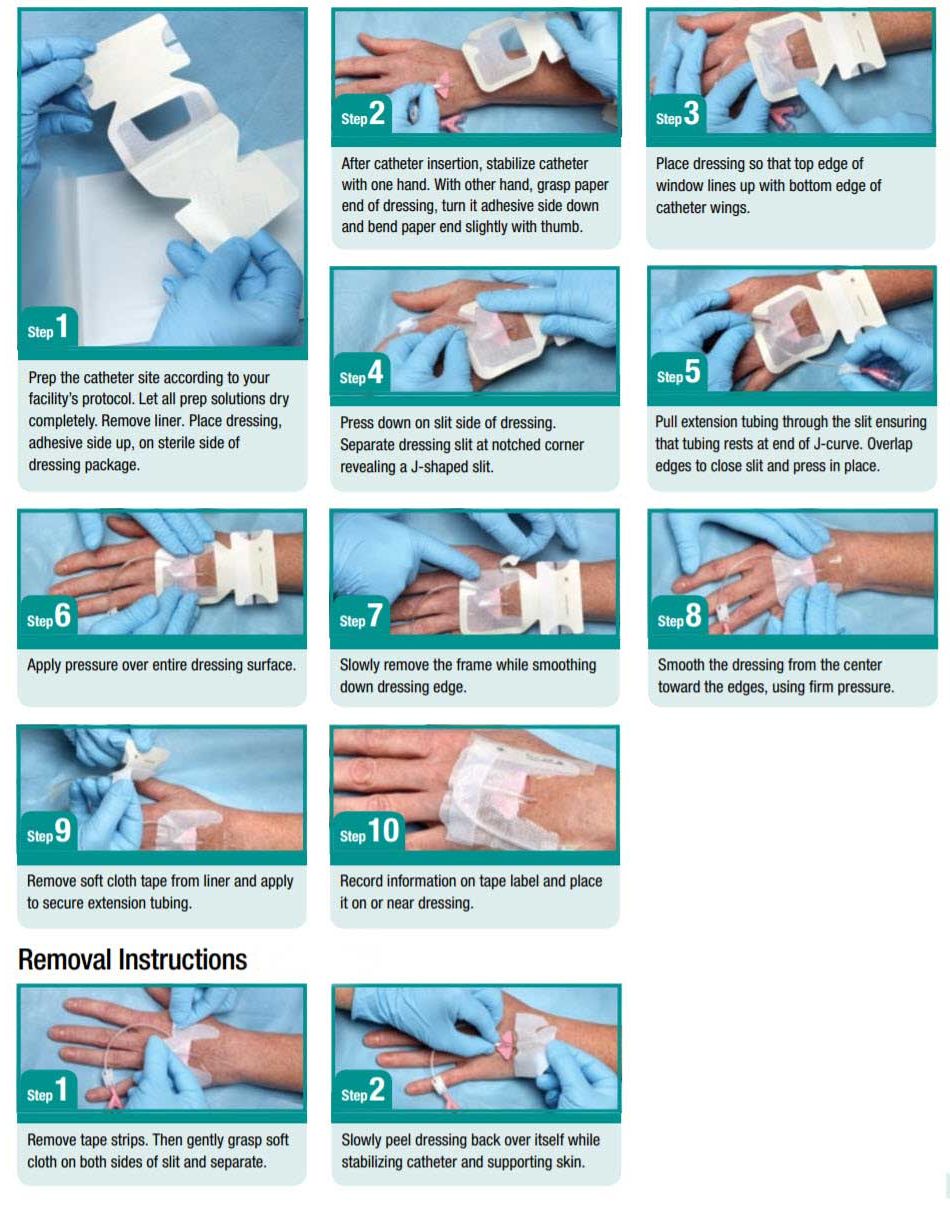
I.V. Dressing Tips
- For added catheter stability, a small strip of non-stretchy tape can be placed over the hub without obscuring the site. If placed under the dressing, use sterile tape.
- For subclavian and jugular sites, apply the dressing with the patients head turned away and neck extended as expected in normal movement. This helps prevent contamination of the site from respiratory secretions and stress on the dressing when the patient moves.
- When preparing a site, always clip excess hair including the beard area to ensure good dressing adhesion. Watch for regrowth, which can lift the dressing off the skin.
- For multilumen, pulmonary artery and dialysis catheters, select bordered/notched dressings. These dressings are designed to prevent dressing lift caused by the weight of the catheter or manipulation of the lumens.
Specifications