Description
3M W8514 - Steri-Strip Sys 1-7/8x1/2" Wound Closur 4x25/CS
3M Steri-Strip Wound Closure System, 2-3/8 in. x 1-7/8 in./60mm x 47mm (Dressing) 7/8 in. x 1/2 in./47mm x 12mm (Closure), 1 Closures/Envelope, 25 Envelopes/Box, 4 Boxes/Cases
Excellent cosmesis with 3M Steri-Strip Skin Closures combined with the bacterial and viral protection of 3M Tegaderm Transparent Film Dressing.
The 3M Steri-Strip Wound Closure System consists of individual skin closure/dressing. Each closure/dressing is composed of one 1/2" Steri-Strip skin closure in combination with an elliptical shaped Tegaderm Transparent Film Dressing. Steri-Strip Adhesive Skin Closures are made of porous, nonwoven material. They are reinforced with filaments for strength and are coated with a hypoallergenic adhesive. Combines the suture and staple-free wound closure of our Steri-Strip products with the sterile, waterproof barrier protection of 3M Tegaderm Transparent Dressings. Allows patients more freedom of movement and the ability to bathe or shower without changing dressings. May be worn up to seven days.
Intended Use: Steri-Strip skin closures are indicated for use as a skin closure device in the treatment of lacerations and surgical incisions. Steri-Strip skin closures may be also used in conjunction with skin sutures and staples or after their removal for wound support.
Using Adhesive Skin Closures Effectively
Which 3M Steri-Strip Skin Closure wound should I use that is less noticeable?
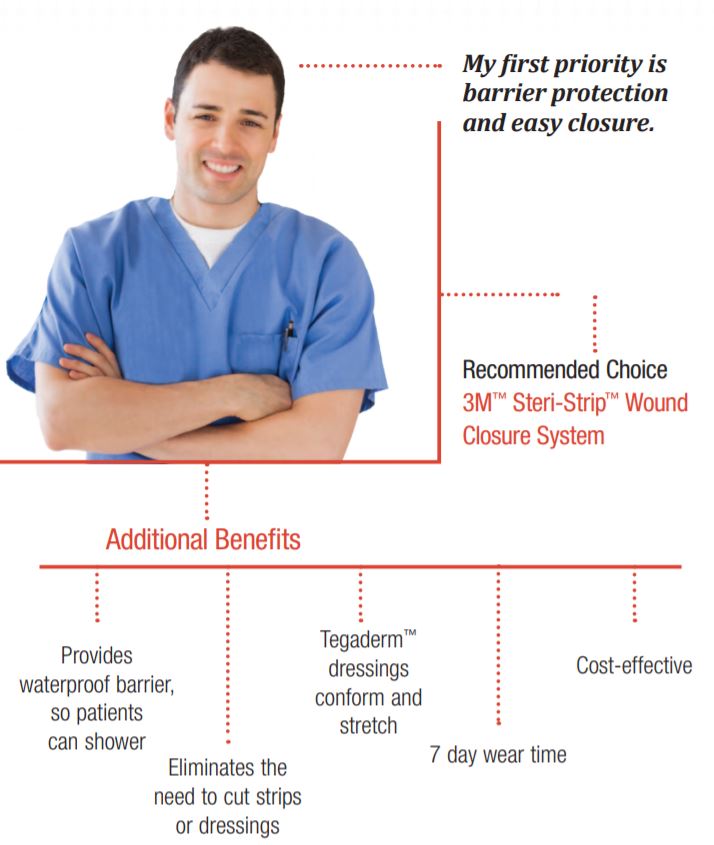
Helps improve cosmetic outcomes
Non-invasive, sterile design helps reduce scarring and the risk of infection, compared to sutures and staples, with less tissue trauma and better cosmetic outcomes. (Reference on file).
Protects
Provides both bacterial and viral protection.*
Easy on patients
Waterproof, yet breathable film barrier enables patients to shower, while allowing more comfort and freedom of movement.
Reduces dressing changes
Breathable closure and dressing may be worn up to 7 days.
Easy to use
Combines quick, easy closure with waterproof barrier protection in one convenient product.
Big Impact. Small Solutions.
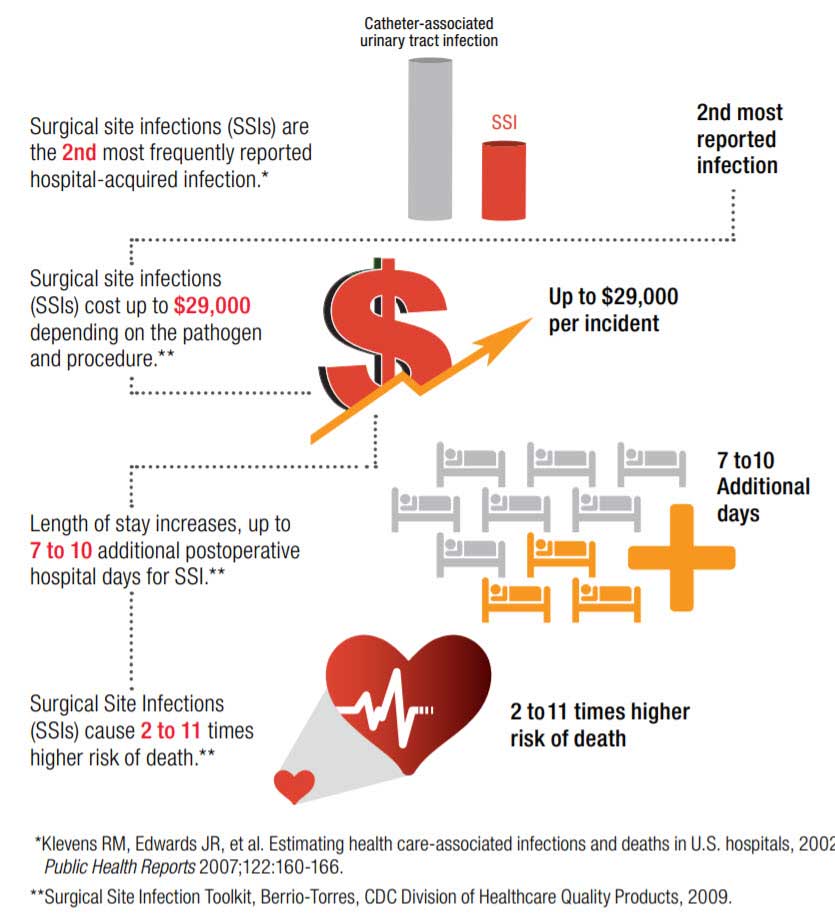
Stopping infections before they start is a big part of your job. Surgical site infections (SSIs) are the second most-common hospital-acquired infection (HAI) accounting for 20% of all HAIs among hospitalized patients,* and costing up to $29,000 depending on the pathogen and procedure.**
Barrier Protection & Easy Closure
In the fight against surgical site infections (SSIs), the combination of a 3M Steri-Strip Reinforced Skin Closure and 3M Tegaderm Transparent Film Dressing is proven to provide a viral* and bacterial
barrier, helping to reduce the risk of infection and set the foundation for improved cosmesis compared to invasive sutures or staples. Better yet, its from 3M - a company you know has it covered when it comes to quality. So you can go about the business of healing with confidence.
Improved Infection Control
Tegaderm Transparent Film Dressings create a transparent, sterile, barrier that is impervious to liquids, bacteria, and viruses*, providing an effective barrier to external contaminants. The adhesive is gentle to the skin, yet has good adherence.
Clear Difference
Unlike gauze dressings, Tegaderm Transparent Film Dressings offer a transparent, waterproof film that serves as a barrier. Tegaderm Transparent Dressings are breathable, letting oxygen in and moisture vapor out, allowing the skin to function normally.
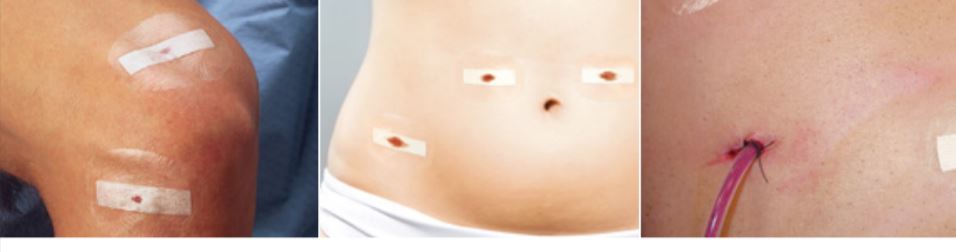
Cosmetic Results
Steri-Strip Skin Closures provide better cosmetic outcome than sutures or staples,1 and are associated with a lower infection rate.
Despite modern advancements in infection prevention, health care-acquired infections (HAIs) remain one of the top 10 leading causes of death in the United States and are responsible for nearly 100,000 deaths each year.1 Surgical site infections (SSIs) are the second most-common HAI, accounting for 20% of all HAIs among hospitalized patients. Post-operative SSIs are the most common healthcare-associated infection in surgical patients occurring in up to 5 percent of surgical patients.

Suggested Applications
Steri-Strip Wound Closure System is used for arthroscopic surgeries and other applications. Ideal for wounds and incisions 2.5cm or less. Same day surgery centers.
- Arthroscopic surgeries
- Laparoscopic surgeries
- Biopsies
- Small surgical incisions
Contraindications
- Steri-Strip skin closures are contraindicated where adhesion cannot be obtained. Potential causes of inadequate adhesion are presence of exudate, skin oils, moisture, or hair.
- Use of Steri-Strip skin closures on infected wounds is contraindicated.
- Steri-Strip skin closures are contraindicated for use in high tension wounds which cannot be easily approximated with finger or forceps.
Warnings
- The development of postoperative edema may cause skin shearing, skin blistering, or loss of tape adhesion to occur at either end of the strip.
- Application of any surgical tape or adhesive skin closure may result in skin stripping upon removal.
- As with all adhesive products applied to the skin, a small percentage of individuals may experience hypopigmentation or hyperpigmentation following removal.
- Occasional cases of mild acne and folliculitis have been observed in testing on healthy volunteers.
- The packaging of this product contains natural rubber latex which may cause allergic reactions.
Precautions
- The skin should be clean, dry, and free of skin oils to assure good adhesion.
- Do not apply skin closures under tension. Skin shearing, skin blistering, or loss of adhesion may result if excessive tension is applied.
3M Steri-Strip Blend Tone Skin Closures(non-reinforced) | Commonly Asked Questions
1. Components
Question: Does the Steri-Strip wound closure system contain latex?
Answer: The Reinforced Steri-Strip skin closures and 3M Tegaderm dressing do not contain natural rubber latex.
However, the current package of this product contains natural rubber latex which may cause allergic reactions.
Question: Do Steri-Strip skin closures contain fiberglass?
Answer: No. Steri-Strips skin closures have never contained fiberglass. The reinforcing filaments in the Reinforced Steri-Strip skin closures in the wound closure system are made of polyester.
Question: Is the Steri-Strip wound closure system hypoallergenic?
Answer: Yes, the Reinforced Steri-Strip skin closures and Tegaderm dressing are hypoallergenic BUT
The Current Package Of This Product Contains Natural Rubber Latex Which May Cause Allergic Reactions.
Question: What is the shelf life of Steri-Strip wound closure system?
Answer: 5 years. The expiration date may be found on the top of each Steri-Strip skin closure package. Without breaking the seal, fold the rear package flap back. Holding the package up to the light, you will note: LOT, hourglass, year, 1-2 numbers, several letters The hourglass symbol represents the expiration date. 2004-10 would indicate that the product will expire in October of 2004.
Question: Is the Steri-Strip Wound Closure System sterile?
Answer: Yes
2. Selection and application
Question: What is the best way to get great adhesion of Steri-Strip skin closures?
Answer: Steri-Strip skin closures adhere best to clean, dry skin. This can be accomplished by ensuring that bleeding is under control and cleaning the skin with saline or alcohol to remove oils or exudates followed by gentle drying of the skin surface. Steri-Strip closures have a "pressure sensitive" acrylate adhesive. Adhesion of skin closures will be enhanced if you gently but firmly press or stroke the skin closure strip during application so that the adhesive gets into the nooks and crannies of the skin surface. See application instructions. Steri-Strip skin closures were designed for closure of low-tension wounds. They are contraindicated for use in high-tension wounds that cannot easily be approximated with fingers or forceps or on infected wounds.
Question: Should I use a skin tackifier under Steri-Strip skin closures?
Answer: Compound benzoin tincture (CBT) may be used to increase adhesion of SteriStrip skin closures. Although, CBT does not contain iodine, it may be an irritant to some individuals and should not enter the wound.
Question: What can I do to protect very fragile skin?
Answer: Apply the skin closure to dry, clean skin that is free of chemicals. If hair is to be removed, a clipper should be used. To further protect from adhesive trauma, a skin barrier such as 3M Cavilon No Sting Barrier Film may be applied and allowed to dry thoroughly before applying the skin closure. Avoid chemicals designed to increase adhesion. To reduce the risk of skin stripping, gently remove Steri-Strip skin closures: "low and slow", towards the center of the wound, while supporting the skin.
Question: What could cause blistering of the skin under the ends of skin closure strips?
Answer: The most common cause of blistering is tension. Edema, hematoma, or distention due to bloating may distort the skin surface. If the adhesive is firmly attached to the epidermis and the adhesive closure backing does not "give", the epidermis may be pulled away from the dermis resulting in blisters or skin tears. Blistering is usually noted on both ends of the strip. A similar situation occurs when a joint or other area of movement is covered with a strip that doesnt stretch. If tension is noted, loosen, reposition, or replace the skin closure strips. To decrease the risk of tension injuries:
- Do not use Steri-Strip skin closures on high-tension wounds.
- Apply Steri-Strip skin closures without tension. Do not strap the wound closed.
- Consider 3M Steri-Strip Elastic Skin Closures if edema, hematoma, distention, or movement are anticipated.
- Monitor the wound for evidence of swelling or blistering.
- If tension is noted under Steri-Strip closures, they may be replaced under the direction of a health care professional.
Question: I suspect that the area around the wound may swell. Will this affect the type of skin closure I should use?
Answer: Yes, if expansion or movement (such as over a joint) is anticipated, consider using Steri-Strip Elastic skin closures.
3. Length of wear
Question: How long can Steri-Strip skin closures be worn?
Answer: Steri-Strip skin closures are usually worn until they fall off or the healthcare provider removes them. This usually occurs within 7 to 14 days for the elastic skin closures. Wear time may vary depending on area of the body, skin type, degree of friction to that area, etc. Some health care providers prefer to remove, and sometimes replace, the SteriStrip skin closures, on follow-up visits. The timing of strip removal and the length of continued reinforcement and support of the wound with skin closure strips is left to the discretion of the health care provider.
Question: If skin closure ends loosen, should they be trimmed?
Answer: Yes. Once the edges loosen, a skin closure strip is more likely come off than if the strip is intact. If the loosened ends of the strips are trimmed so that they do not catch on clothing, the duration of adherence is extended. However, when less than 1/2 inch of the strip remains on either side of the wound, health care providers may want to consider gently removing the strip and replacing it with a new strip.
Question: Can I get Steri-Strip skin closures wet?
Answer: Unless contraindicated, you may shower 24 hours after the Steri-Strip skin closures have been applied. Then pat the strips dry. However, some studies indicate that strip adherence may be improved when bathing is limited. For additional protection, you may cover the Steri-Strip skin closure with a 3M Tegaderm Transparent Dressing.
Wound Closure Application

Specifications