Description
Product Description
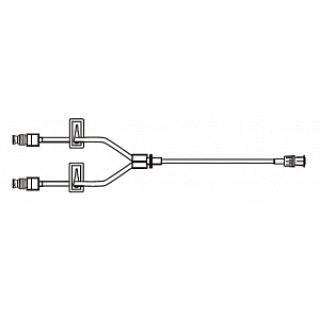
Alaris Medical 20019E - SmartSite extension set pressure rated smallbore bifuse, 2 SmartSite needle-free connector(s), 2 slide clamp(s), spin male luer lock, 100/CS
Alaris Medical 20019E SmartSite extension set pressure rated smallbore bifuse
SmartSite needle-free valve and other SmartSite devices provide a wide variety of benefits in IV therapy including helping prevent infection. Free of needles, they reduce the risk of bloodborne needlestick injuries. They feature a smooth, swabbable surface that you can disinfect at every access point. Standard with administration, gravity, secondary and extension sets, our SmartSite technology is designed to enhance safety for both you and your patients.
- Not made with DEHP. L: 9.5 in L: 24 cm PV: 0.4 mL fluid path sterile
- Labeled for use with low pressure power injectors up to 325 PSI and maximum flow rate of 10 mL/second.
Device Characteristics of Alaris Medical 20019E SmartSite extension set
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): |
No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |