Description
Avanos Medical 0100-14LV - MIC Gastrostomy Feeding Tube, 14 Fr, 5 EA/CS
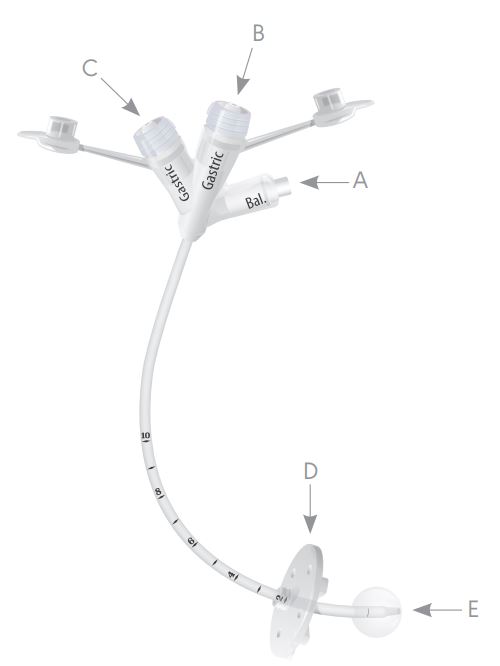
The AVANOS 0100-14LV MIC Gastrostomy Feeding Tube delivers optimal performance and value for anyone requiring gastrostomy tube. The unique design of the MIC G Feeding Tube extends the balloon beyond the distal tip at recommended fill volumes, thereby decreasing the potential for gastric wall irritation or erosion. The tip is tapered, allowing for an easier insertion and an enhanced patient experience. Practical features for ease of use: The MIC G is made with medical-grade silicone to aide with visibility. The MIC G is equipped with a luer slip balloon port connector for a more efficient one-handed operation.
The AVANOS 0100-14LV MIC Gastrostomy Feeding Tube allow for delivery of enteral nutrition and medication directly into the stomach and/or gastric decompression.
AVANOS 0100-14LV MIC Gastrostomy Feeding Tube Features
- Medical grade silicone construction
- Inflatable silicone internal retention balloon
- SECUR-LOK external retention ring
- Tapered or non-tapered distal tip
- Radiopaque stripe
About AVANOS 0100-14LV MIC Gastrostomy Feeding Tube
The AVANOS 0100-14LV MIC Gastrostomy Feeding Tube are held in place by an internal retention balloon. Water was injected into the balloon through the balloon inflation port. The maximum fill volume of the balloon is printed on the balloon inflation port. The balloon rests against the inside of your stomach wall.
|
 |
AVANOS 0100-14LV MIC Gastrostomy Feeding Tube Maintenance and Stoma Care
Indications for Use
The AVANOS 0100-14LV MIC Gastrostomy Feeding Tube are indicated for use in patients who require long term feeding, are unable to tolerate oral feeding, who are at low risk for aspiration, require gastric decompression and / or medication delivery directly into the stomach.
Contraindications
Contraindications for placement of a gastrostomy feeding tube include, but are not limited to ascites, colonic interposition, portal hypertension, peritonitis and morbid obesity.
Complications
The following complications may be associated with any low-profile gastrostomy feeding tube:
- Skin Breakdown
- Infection
- Hypergranulation Tissue
- Stomach or Duodenal Ulcers
- Intraperitoneal Leakage
- Pressure Necrosis
Note: Verify package integrity. Do not use if package is damaged or sterile barrier compromised.
Placement
The AVANOS MIC Gastrostomy / Bolus feeding tubes may be placed surgically, percutaneously under fluoroscopic or endoscopic guidance or as a replacement to an existing device using an established stoma tract.
AVANOS 0100-14LV MIC Gastrostomy Feeding Tube Daily Care & Maintenance Check List
Assess the patient: Assess the patient for any signs of pain, pressure or discomfort.
Assess the stoma site: Assess the patient for any signs of infection, such as redness, irritation, edema, swelling, tenderness, warmth, rashes, purulent or gastrointestinal drainage. Assess the patient for any signs of pressure necrosis, skin breakdown or hypergranulation tissue.
Clean the stoma site:
- Use warm water and mild soap.
- Use a circular motion moving from the tube outwards.
- Clean sutures, external bolsters and any stabilizing devices using a cottontipped applicator.
- Rinse thoroughly and dry well.
Assess the tube: Assess the tube for any abnormalities such as damage, clogging or abnormal discoloration.
Clean the feeding tube:
- Use warm water and mild soap being careful not to pull or manipulate the tube excessively.
- Rinse thoroughly, dry well.
Clean the gastric and balloon ports: Use a cotton tip applicator or soft cloth to remove all residual formula and medication.
Rotate the tube: Rotate the tube 360 degrees plus a quarter turn daily.
Verify placement of the external bolster: Verify that the external bolster rests 23 mm above the skin.
Flush the feeding tube: Flush the feeding tube with water using a catheter tip or slip tip syringe every 4-6 hours during continuous feeding, anytime the feeding is interrupted, or at least every 8 hours if the tube is not being used. Flush the feeding tube after checking gastric residuals. Flush the feeding tube before and after medication administration. Avoid using acidic irrigants such as cranberry juice and cola beverages to flush feeding tubes.
AVANOS 0100-14LV MIC Gastrostomy Feeding Tube Balloon Maintenance
Check the water volume in the balloon once a week.
- Insert a Luer slip syringe into the balloon inflation port and withdraw the fluid while holding the tube in place. Compare the amount of water in the syringe to the amount recommended or the amount initially prescribed and documented in the patient record. If the amount is less than recommended or prescribed, refill the balloon with the water initially removed, then draw up and add the amount needed to bring the balloon volume up to the recommended and prescribed amount of water. Be aware as you deflate the balloon there may be some gastric contents that can leak from around the tube. Document the fluid volume, the amount of volume to be replaced (if any), the date and time.
- Wait 10-14 minutes and repeat the procedure. The balloon is leaking if it has lost fluid, and the tube should be replaced. A deflated or ruptured balloon could cause the tube to dislodge or be displaced. If the balloon is ruptured, it will need to be replaced. Secure the tube into position using tape, then follow facility protocol and/or call the physician for instructions.
Note: Refill the balloon using sterile or distilled water, not air or saline. Saline can crystallize and clog the balloon valve or lumen, and air may seep out and cause the balloon to collapse. Be sure to use the recommended amount of water as over-inflation can obstruct the lumen or decrease balloon life and underinflation will not secure the tube properly
AVANOS 0100-14LV MIC Gastrostomy Feeding Tube Device Characteristics
| What MRI safety information does the labeling contain? | MR Safe |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | No |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
