Description
Product Description
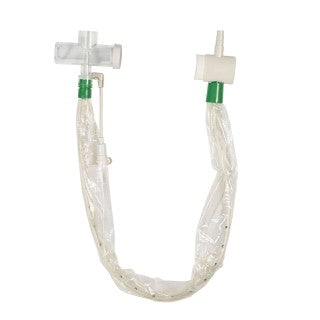
Avanos Medical 2155 - BALLARD Closed Suction System, Adults, 12 FR, T-Piece, 20/Case
Avanos Medical 2155 - BALLARD Closed Suction System
Leader in closed suctioning for more than 30 years, BALLARD Standard Closed Suction Systems are a powerful tool in your fight against VAEs. They are designed to remove secretions from the airway while maintaining ventilation and oxygen therapy throughout the suctioning procedure. Closed-suction systems reduce the potential risk for the spread of infection and contamination entering the airway and protects the caregiver from cross-contamination.
Avanos Medical 2155 - BALLARD Closed Suction SystemFeatures & Benefits
- Reusable up to 24 hours
- Soft low durometer catheter to minimize tracheal trauma
- Tactile sleeve for catheter advancement
- Integrated MDI Port (Optional)
Avanos Medical 2155 - BALLARD Closed Suction System Specifications
| Diameter (Fr) | 12 |
| Made With DEHP | No |
| Single Use | True |
| Sterile | Yes |
| Sterilization Method | GAMMA |
| Catheter Length (In.) | 21.25 |
| MDI Attachment | No |
| Primary Product Color | White |
| Product Brand | BALLARD |
| Kit, Pack, or Tray | False |
Avanos Medical BALLARD Closed Suction System 2155 Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as ""Not made with natural rubber latex"": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | No |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |