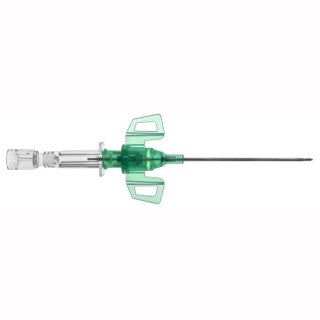
Description
Product Description
B Braun 4251132-02 - Introcan Safety 3 Closed IV Catheter 18 Ga. x 1.75 in., PUR, Winged, 200/CS
B Braun 4251132-02 Introcan Safety 3 Closed IV Catheter
The FIRST fully automatic passive safety peripheral IV catheter with needlestick AND multiple access blood exposure protection that cannot be bypassed.
| Catalog No. | Gauge | Length | Gravity Flow Rate (mL/min) |
| 4251132-02 | 18 | 1.75 in. (45 mm) | 95 |
| Contrast Media Viscosity (mPa*s) | Flow Rate mL/sec) | Material | Packaging |
| 2.3 27.5 |
19 15 |
PUR | 200/CS |
Automatically control blood exposure
- Reduce exposure to blood
- Helps reduce clean-up time and materials
Automatically prevents needlesticks
- Passive safety shield automatically engages and cannot be bypassed
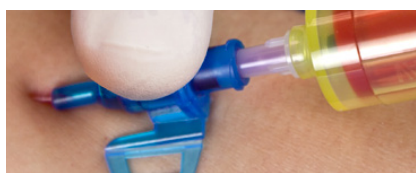
Multiple access blood control
For blood collection, boluses or set changes without blood exposure
 |
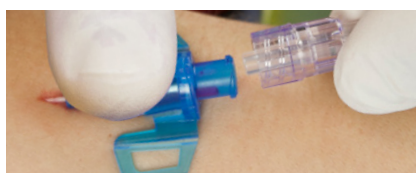 |
| Draw blood | Connect extension set |
Confidence and Peace of Mind for You
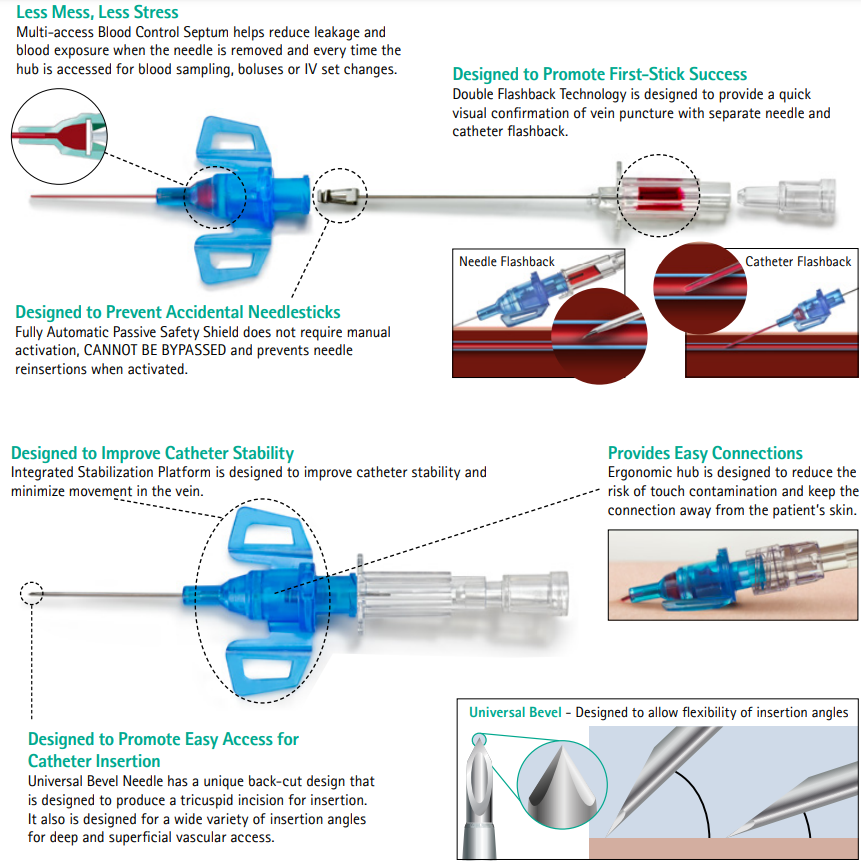
B Braun 4251132-02 Introcan Safety 3 Closed IV Catheter insertion guide
Preparation
- Select and prepare site according to facility protocol.
- Completely remove the paper from the packaging.
- Flex wings up and down multiple times.
- Remove protective cover by holding at each end, then pull straight apart.
- DO NOT ROTATE CATHETER PRIOR TO INSERTION
- Confirm catheter hub is seated tightly against flashback chamber.
Perform insertion
- Hold skin taut, insert catheter at optimal insertion angle.
- Visualize first flashback in flashback chamber to confirm needle entry in the vessel.
- Upon first flashback visualization, LOWER and advance the needle and catheter together approx 3mm or 1/8in. to ensure catheter tip is in the vessel.
Thread catheter
- Holding needle still, advance the catheter off needle and visualize second flash within the catheter to confirm catheter is in the vessel.
- After confirmation, continue advancing catheter off the needle into the vessel.
- Release tourniquet
Stabilize catheter hub and remove needle
- With hub stabilized, swiftly remove needle straight out from hub.
- The passive safety shield automatically covers the needle bevel.
- Properly discard needle into sharps container
Connect and secure catheter
- Immediately CONNECT and TIGHTEN the accessory device to the catheter hub.
- Stabilize and dress the site per facility protocol while maintaining proper hub angle.
Device Characteristics of B Braun 4251132-02 Introcan Safety 3 Closed IV Catheter
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): |
No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |