Description
Product Description
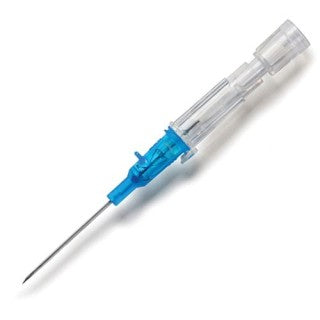
B Braun 4251628-02 - Introcan Safety IV Catheter 22 Ga. x 1 in., PUR, Polyurethane catheter material, straight hub, 200/CS
B Braun 4251628-02 Introcan Safety IV Catheter 22 Ga. x 1 in., PUR
This easy-to-use safety IV catheter incorporates separate needle and catheter flashback - Double Flashback technology - that gives you the added confidence of successful placement every time. The sharp needle features a Universal Bevel geometry that allows a wider choice of insertion angles, which is designed to make the catheter easy to adapt to and venipuncture more comfortable for patients. And with less plastic content than other safety IV catheters, you can potentially reduce storage space and medical waste.
Device Characteristics of B Braun 4251628-02 Introcan Safety IV Catheter 22 Ga. x 1 in., PUR
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): |
No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |