Description
B Braun 4252535-02 - Introcan Safety IV Catheter 20G x 1.25 FEP (S), 200/CS
B Braun 4252535-02 Introcan Safety IV Catheter
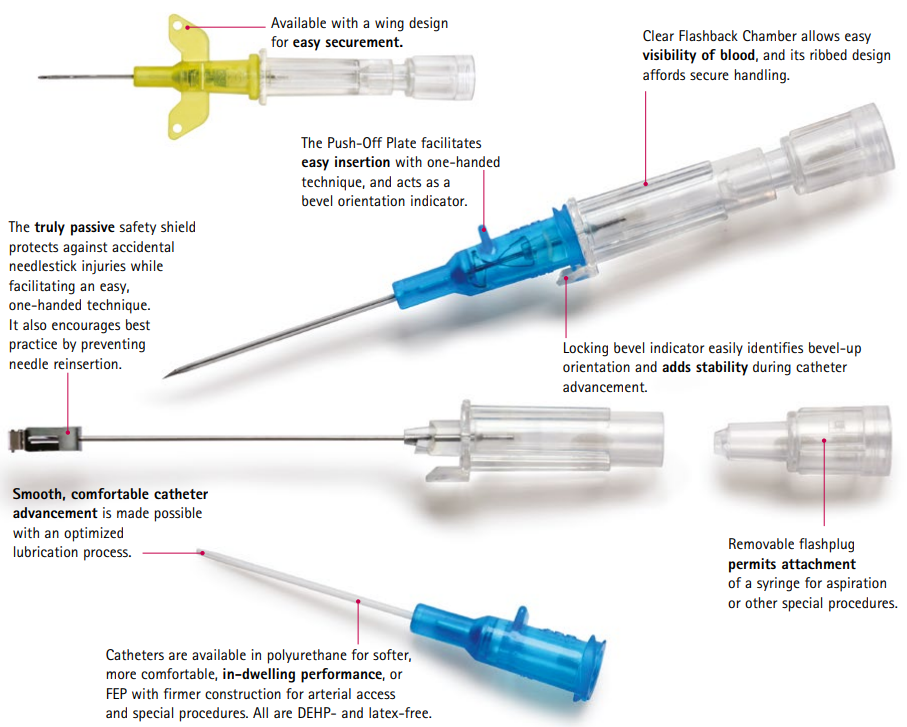
Although safety devices were introduced over a decade ago, preventing needlestick injuries continues to be a major concern. With the B. Braun Introcan Safety IV Catheter, you are protected by a truly passive safety device that is activated automatically and cannot be by-passed. No extra steps are required to ensure healthcare worker and patient safety.
This easy-to-use safety IV catheter incorporates separate needle and catheter flashback - Double Flashback technology - that gives you the added confidence of successful placement every time. The sharp needle features a Universal Bevel geometry that allows a wider choice of insertion angles, which is designed to make the catheter easy to adapt to and venipuncture more comfortable for patients. And with less plastic content than other safety IV catheters, you can potentially reduce storage space and medical waste.
Raise your facilitys standard of care and choose the B. Braun Introcan Safety IV Catheter to:
- Reduce Needlestick Injuries: recent studies1,2 confirm that passive safety designs offer 2-3 times better protection against accidental needlesticks than active designs. INS Standards also recommend the use of passive safety devices.
- Promote First Stick Success: the innovative Double Flashback technology allows separate confirmation of both needle and catheter placement, which can result in better first stick success and greater clinician confidence. New technology in our 24 Ga. catheters is designed to speed flashback of blood to help confirm placement in patients. Research also shows Introcan Safety is easier to introduce into the vein compared to the leading IV catheter.
- Improve Patient Comfort: the sharp needle features a Universal Bevel that is designed for a wider choice of insertion angles and produces less tearing.
- Ensure Best Practices: the passive safety design ensures that the safety mechanism is always activated and prevents re-insertion of the stylet. Additionally, a recent study shows Introcan Safety results in less blood exposure to staff than catheters featuring spring-retraction of the needle.
- Help the Environment: lighter, more compact design can potentially lower shipping, storage and waste disposal costs.
B Braun 4252535-02 Introcan Safety IV Catheter Insertion Guide
Preparation
- Select and prepare site according to facility protocol.
- Completely remove the paper from the packaging.
- Remove protective cover by holding at each end, then pull straight apart.
- DO NOT ROTATE CATHETER PRIOR TO INSERTION
- Confirm catheter hub is seated tightly against flashback chamber.
Perform insertion
- Hold skin taut, insert catheter at optimal insertion angle.
- Visualize first flashback in flashback chamber to confirm needle entry in the vessel.
- Upon first flashback visualization, LOWER and advance the needle and catheter together approx 3mm or 1/8 in. to ensure catheter tip is in the vessel.
Thread catheter
- Holding needle still, advance the catheter off needle and visualize second flash within the catheter to confirm catheter is in the vessel.
- After confirmation, continue advancing catheter off the needle into the vessel.
- Release tourniquet.
Occlude vessel and stabilize catheter hub
Remove needle from catheter
- With hub stabilized, swiftly remove needle straight out from hub.
- The passive safety shield automatically covers the needle bevel.
- Properly discard needle into sharps container
Connect and secure catheter
- Immediately CONNECT and TIGHTEN the accessory device to the catheter hub.
- Stabilize and dress the site per facility protocol.
Device Characteristics of B Braun 4252535-02 Introcan Safety IV Catheter
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |