Description
Product Description
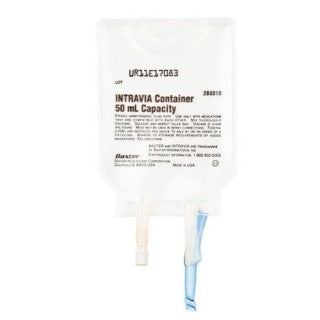
Baxter 2B8019 - Intravia Container Empty 50mL 48/CS
Empty INTRAVIA Container with PVC Ports, Sterile fluid path, 50 mL
Sterile Nonpyrogenic fluid path use only with medications that are compatible with each other mix thoroughly.
INTRAVIA Plastic Container systems
- INTRAVIA Container, 50 mL Capacity
- The solution contact materials in empty 2J INTRAVIA Container
- Contain DEHP
- Radiation - Gamma sterilized
Cautions
- Squeeze and inspect filled bag
- Discard if leaks are found
- Must not be used in series connections
Contains DEHP
Diethyl hexyl Phthalate, A chemical commonly used in PVC tubing; used to yield clarity and soft pliable tubing.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
Sterilization
| Device Packaged as Sterile: | Yes |
| Requires Sterilization Prior to Use: | No |
Product Specifications
| Manufacturer No. | 2B8019 |
| Brand | Intravia |
| Manufacturer | Baxter |
| Latex | Not Made with Natural Rubber Latex |
| Application | Medication Delivery Bag (empty) |
| Capacity | 50 mL |
| Material | Polyolefin |
| Port Type | 2 - PVC Ports |
| DEHP | Contains DEHP |
| Concentration | Rx Only. |
Packing Information
| Length: | 9.75 IN |
| Width: | 9.75 IN |
| Height: | 6 IN |
| Volume: | 0.33 FC |
| Weight: | 1.3 LB |
| Pack Factor: | 48 |