Description
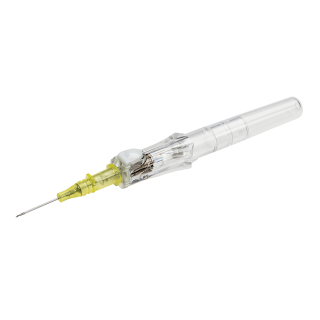
BD 381511 - Insyte-N Autoguard shielded IV catheter, Yellow, Non Winged, 50/Box, 4 Box/CS

BD 381511 Insyte Autoguard BC Shielded IV Catheter
The BD Insyte-N Autoguard shielded IV catheter includes all of the features of the BD Insyte Autoguard shielded IV catheter in a 0.56-in catheter length. With the push of a button, the needle instantly retracts - reducing the risk of accidental needlestick injuries. The catheter is also made with BD Vialon biomaterial, which is designed to recover after kinking to help provide longer dwell times and therefore, minimize restarts.
Push-button shielding |
Improved first-stick success |
BD Vialon biomaterial |
| BD push-button needle shielding technology is demonstrated to reduce needlestick injuries by 95%. | BD Instaflash needle technology on the catheter''s 20-G, 22-G and 24-G sizes incorporates a notched needle that may improve first-stick success and reduce painful hit-and-miss insertions. | This unique proprietary biomaterial softens up to 70% in the vein, enabling longer dwell times and reducing the chance of mechanical phlebitis by up to 50%. |
| Catalog No. |
Color | Gauge | Length (in) |
| 381511 | Yellow | 24 | 0.56 in. |
| Catheter ID (mm) | Catheter OD(mm) | Gravity Flow Rate (mL/min) | High Pressure Rating (psi) |
| 0.521 | 0.699 | 20 | N/A |
|
Proven to optimize patient outcomes Multiple clinical investigations conducted over ten years have proven that IV catheters made of BD Vialon biomaterial are easier to insert, while providing longer indwelling times with improved patient outcomes. ..catheters made of PEU-Vialon were substantially less phlebitogenic than were catheters made of FEP-Teflon. The reduction in risk was nearly 30% overall; in severe phlebitis, the reduction was nearly 50%. |
 |
|
Designed to increase first-stick success. A successful first-stick is important for both you and your patient, especially when accessing small and compromised veins. BD Instaflash needle technology is designed to reduce hit-and-miss insertion by immediately confirming vessel entry through a unique notch in the needle shaft. BD Medical provides the largest selection of notched needle devices and has incorporated its notched needle technology into our 20, 22, and 24-gauge BD Insyte Autoguard shielded IV catheters.
|
Proven to effectively reduce needlesticks. BD Insyte Autoguard shielded IV catheters mean added safety for you. Theyre the safety-engineered version of the popular BD Insyte IV catheter. The patented BD Autoguard push-button shielding technology is proven to effectively safeguard healthcare workers from accidental needlestick injuries... "...the safety IV catheter (Insyte Autoguard) resulted in a marked and significant reduction in IV styletrelated injuries..." |
BD 381511 Insyte Autoguard BC Shielded IV Catheter Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |