Description
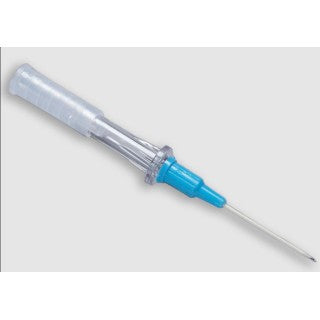
BD 382277 - IV Angiocath Catheter 12Gx3 10/BX
BD 382277 Angiocath IV Catheter
BD Angiocath Peripheral IV Catheters are designed for smooth insertion. They are made with FEP (Fluorinated Ethylene Propylene) polymer. FEP (Fluorinated Ethylene Propylene) polymer catheter material features a thin-wall design and a tapered tip that results in a smooth insertion and optimal flow rates.
- Made of radiopaque FEP polymer
- Thermo-moulded bevel ensures excellent penetration properties
- Recommended for infusion, transfusion, angiography
- Triple-bevel needle
- Transparent clear flashback chamber
- Available in longer lengths (14 G and 16 G available with 83 mm and 133 mm long catheters)
- Diameters 12 G and 10 G permit very large volume emergency infusions
- BD Luer-Lok compatible
- Sterile, single use
- Disposable to help reduce cross contamination
- Available in longer lengths for special placement (3.00, 3.25, and 5.25 inches)
BD 382277 Angiocath IV Catheter Specifications
| Catalog No. | Color | Gauge | Catheter Length (in) | Catheter ID (mm) | Catheter OD (mm) |
Gravity Flow Rate (mL/min) |
Packaging |
| 382277 | Pale Blue | 12 | 3.00 | 2.235 - 2.337 | 2.692 - 2.896 | 449 | 10/Box 50/Case |
What is Angiocath IV Catheter
A sterile, thin, flexible tube intended to be inserted into the peripheral vasculature of a patient to enable short-term (< 30 days) intravascular access; it is not intended to be advanced to the central vasculature. It typically includes dedicated accessories to facilitate catheter introduction/placement and function (e.g., connectors, injection ports, stylet and/or wings for fixation). It may be used for blood sampling, monitoring of blood pressure, to administer fluids, medication and/or for the injection of contrast media. This is a single-use device. AcessGUDID
BD 382277 Angiocath IV Catheter Device Characteristics
| What MRI safety information does the labeling contain? | MR Conditional |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |