Description
Product Description
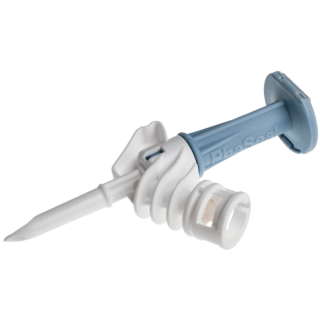
BD 515306 - PhaSeal Infusion Adapter C100, 50/Box, 200/CS
BD 515306 PhaSeal Infusion Adapter C100
BD PhaSeal IV bag and line access devices attach the IV bag to the IV line, and the BD PhaSeal syringe safety device-syringe assembly to IV bag to form an airtight and leak-proof connection for transferring and administering the drug within a closed system.
Specifications of BD 515306 PhaSeal Infusion Adapter C100
| Manufacturing Site | Spain |
| DEHP | DEHP-Free |
| Latex | Latex-Free |
| Sterilization | Ethylene Oxide |
| Bisphenol A (BPA) | BPA-Free |
| Shelf Life | 4 Years |
| Classification | IIa |
| Quantity per Box | 50 |
| Quantity per Case | 200 |
| Dimensions (mm) | 90 x 24 x 43 |
| Configuration 1 | Single port |
| Configuration 2 | Y-site |
| Type of Access Device | Connector Luer Lock |
| Safety Engineered Feature | Closed system drug transfer |
| Internal Volume (mL) | 0.16 |
| Protective Cover (Material) | Polypropylene (PP) |
| Spike Housing (Material) | Polypropylene (PP) + white colorant |
| Membrane (Material) | Thermoplastic elastomer (TPE) |
| Spike Port (Material) | Thermoplastic Vulcanisates (TPV) |