Description
C.R. Bard 0600620 - HICKMAN Central Venous Catheter, Dual Lumens, Peel-Apart Introducer, 12 Fr, 90cm, EACH
C.R. Bard 0600620 HICKMAN Central Venous Catheter
The HICKMAN Central Venous Catheters form an extensive line of vascular access devices for long-term care.
C.R. Bard 0600620 HICKMAN Central Venous Catheter Features and Benefits
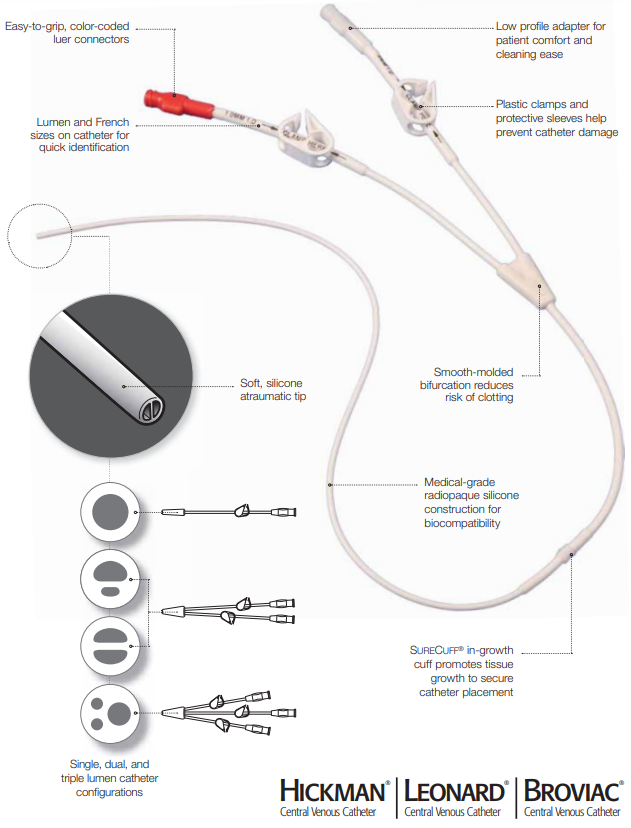
Versatile and Effective
- Single, dual, and triple lumen options help increase the flexibility and efficiency of your treatment
- Available with percutaneous introducer kit to facilitate placement
- Time-saving repair kits extend catheter life
- Silicone construction for short or long-term use
- Soft, atraumatic tip
C.R. Bard 0600620 HICKMAN Central Venous Catheter Indications For Use
HICKMAN are designed for longterm vascular access and for use in patients that lack adequate peripheral venous access. They are available in single, dual and triple lumen catheters. All HICKMAN central venous catheters are designed for the administration of I.V. fluids, blood products, drugs, and parenteral nutrition solutions, as well as blood withdrawal. Note: While smaller lumen Broviac* catheters have been used successfully for blood withdrawal, their small lumen sizes increase the chance of clotting. Contraindications, Warnings, Cautions and Precautions Contraindications The device is contraindicated whenever.
- The presence of device related infection, bacteremia, or septicemia is known or suspected.
- The patients body size is insufficient to accommodate the size of the implanted device.
- The patient is known or is suspected to be allergic to materials contained in the device.
- Severe chronic obstructive lung disease exists (percutaneous subclavian placement only.)
- Past irradiation of prospective insertion site.
- Previous episodes of venous thrombosis or vascular surgical procedures at the prospective placement site.
- Local tissue factors will prevent proper device stabilization and/or access.
Device Characteristics of C.R. Bard 0600620 HICKMAN Central Venous Catheter
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | Yes |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
