Description
Cook Medical COKG18665 - Catheter Sonohysterography Goldstein 5.2Fr 36cm LF10/Bx

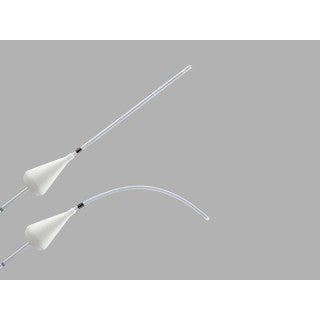
Goldstein Sonohysterography Catheter PTFE
The Goldstein Sonohysterography Catheter is used to access the uterine cavity for sonohysterography. It is available in either PTFE or polyethylene material.
Used to access the uterine cavity for sonohysterography. The adjustable acorn positioner assists in maintaining the catheters seal at the external cervical os. The ink band provides a reference mark to promote proper catheter positioning. Large oval sideport facilitates instillation of saline solution. Intended for one-time use.
| Order Number | Reference Part Number | Fr | Length (cm) |
| G18665 | J-GSHC-523620 | 5.2 | 36 |
- The adjustable acorn-shaped positioner maintains catheter placement while reducing the leakage of instilled saline at the external cervical os.
- An ink band provides a reference mark to indicate that proper catheter positioning has been achieved.
- The round, closed catheter tip eases the introduction of the catheter into the uterus.
- The large oval sideport facilitates the instillation of saline solution.
- The formable polytetrafluoroethylene (PTFE) versions maintain their shape to aid in placement.
Versatility
Formable material maintains the catheter shape for ease of placement.
Efficiency
The rounded tip eases passage of the catheter through the cervix. A large sideport helps with the instillation of saline.
Control
The signature acorn-shaped positioner occludes the cervix to reduce the loss of instilled saline during the procedure.
Contraindications
This device should not be used in the presence of hemorrhage, active pelvic infection, sexually transmitted disease, profuse bleeding, or pregnancy. This device should only be used by an experienced clinician.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
Suggested Instructions for Using Goldstein Sonohysterography Catheter
- Pre-fill catheter with saline and remove syringe prior to placement.
NOTE:Syringe is reattached after catheter is positioned. - With cervix exposed, insert catheter into uterus using packing forceps.
NOTE:Reference mark on catheter may be used to aid in positioning. - Advance positioner into cervix using forceps.
- Instill saline through catheter into uterus.
- Catheter is removed upon completion of ultrasound procedure.
NOTE:Upon removal of catheter, ensure that positioner is still on body of catheter. If still lodged in patient cervix, remove using forceps.
CAUTION:Sterile if the package is unopened or undamaged. Do not use if package is broken.
CAUTION:Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.