Description
Product Description
Cook Medical G16740 - CATHETER CYSTOSTOMY SET 12FR 54CM, EACH
Cook Cystostomy Catheter Set
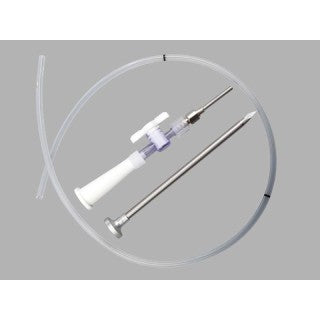
Used for temporary suprapubic urinary diversion and drainage. The stylet is used to puncture the bladder. The access sheath is left in place to ease catheter placement. After the catheter is introduced, the access sheath is removed and discarded. A soft, low-profile catheter support is included for external catheter retention. Set includes trocar stylet with access sheath, catheter, drainage adapter, catheter support, and pull tie.
| Order Number | Reference Part Number | Fr | Length (cm) |
| G16740 | 083312 | 12.0 | 54 |
Components
- Catheter - Silicone
- Drainage adapter with stopcock - Plastic and stainless steel
- Soft low-profile catheter support with pull tie - Silicone
- Trocar stylet and access sheath - Stainless steel
Suggested Instructions for Using Cook Cystostomy Catheter
- Fill the bladder with sterile saline solution if not already distended with urine.
- Use stylet/access sheath to puncture bladder.
- If bladder pressure is low and urine fails to flow spontaneously through the stylet handle, aspirate urine to ascertain whether the stylet/access sheath is within the bladder.
- Leaving access sheath in place, remove stylet.
- Insert silicone catheter (distal tip has sideports) through access sheath to desired location.
- Remove and discard access sheath.
- Attach drainage adapter to catheter.
- Using the pull tie, attach the catheter support to the catheter shaft 1 to 2 cm from the skin surface.
Use suture or surgical tape to fix the catheter support in position and prevent the catheter from being accidentally displaced forward.
CAUTION: Sterile if the package is unopened or undamaged. Do not use if package is broken.
CAUTION: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Sterilization
| Device Packaged as Sterile: | Yes |
| Requires Sterilization Prior to Use: | No |
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |