Description
Cook Medical G22556 - SPHINCTEROTOME, TRIPLE LUMEN, TRI-30, EACH
Tri-Tome pc Triple Lumen Sphincterotome
This device is used for endoscopic cannulation of the ductal system and for sphincterotomy. Precurved DomeTip is designed with anatomy in mind for smooth, easy access.
| Order Number | Reference Part Number | Catheter Size Fr | Tip Shape | Catheter Length (cm) |
| G22556 | TRI-30 | 7 | DomeTip | 200 |
Benefit and Features
Used for endoscopic cannulation of the ductal system and for sphincterotomy.
- DomeTip is designed with anatomy in mind for smooth, easy access
- Custom 3-D forming wire for orientation
- Protector insulation on proximal portion of cutting wire decreases incidence of cutting wire contact with scope, reducing the risk of damage to the cutting wire and scope (available on TRI-25M-P)
Intended Use
This device is used for cannulation of the ductal system and for sphincterotomy.
Notes: Do not use this device for any purpose other than stated intended use. Store in a dry location, away from temperature extremes. Triple lumen feature allows wire guide access to desired duct during sphincterotomy and allows contrast injection through a separate lumen. Use of this device restricted to a trained healthcare professional.
Contraindications
Contraindications include those specific to ERCP and any procedures to be performed in conjunction with sphincterotomy. Contraindications to sphincterotomy include, but are not limited to: coagulopathy and inability to properly position the sphincterotome cutting wire.
Potential Complications
Potential complications associated with ERCP include, but are not limited to: pancreatitis, cholangitis, aspiration, perforation, hemorrhage, infection, sepsis, allergic reaction to contrast or medication, hypotension, respiratory depression or arrest, cardiac arrhythmia or arrest.
Precautions
Refer to package label for minimum channel size required for this device. Maximum rated input voltage for this device is 1.5 kVp-p. Any electrosurgical accessory constitutes a potential electrical hazard to patient and operator. Possible adverse effects include, but are not limited to: fulguration, burns, nerve and/or muscle stimulation and cardiac arrhythmia. Before using this device, follow recommendations provided by electrosurgical unit manufacturer to ensure patient safety through proper placement and utilization of patient return electrode. Ensure a proper path from patient return electrode to electrosurgical unit is maintained throughout procedure. Switch electrosurgical unit to off position when it is not in use. When applying current, ensure cutting wire is completely out of endoscope. Contact of cutting wire with endoscope may cause grounding, which can result in patient injury, operator injury, a broken cutting wire, and/or damage to endoscope. If a non-protected wire guide is used in sphincterotome, it must be removed prior to applying electrosurgical current. Do not over flex or bow tip beyond 90, as this may damage or cause cutting wire to break. Elevator should remain open/down when advancing or retracting sphincterotome.
System Preparation
- Upon removing device from package, uncoil and straighten sphincterotome. Carefully remove precurved stylet from cannulating tip.
Note: Do not apply manual pressure to tip or cutting wire of sphincterotome to influence orientation, as this may result in damage to device.
Note: Do not exercise handle while device is coiled or precurved stylet is in place, as this may cause damage to sphincterotome and render it inoperable. - With electrosurgical unit off, prepare equipment. Active cord fittings should fit snugly into both device handle and electrosurgical unit.
Instructions for Use
For Tri-Tome pc:
- Introduce tip of device into accessory channel and advance in short increments until tip is endoscopically visible.
Note: This device may be placed over a pre-positioned wire guide; prior to doing so, flush wire guide lumen.
Then Refer to Steps 2-5 Below
For Tri-Tome pc Select:
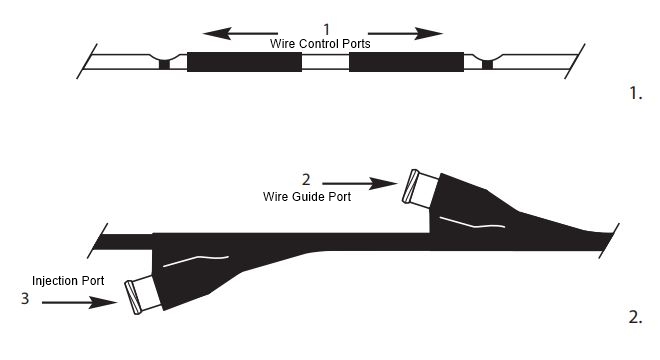
- If using a wire control port, introduce tip of device into accessory channel and advance in short increments until it is endoscopically visible. A .035-inch wire guide may be introduced through desired wire control port. (See Fig. 1)
Note: To open a wire control port, slide cover tubing to expose port. Caution: Keep wire control ports closed when not in use to prevent fluid leakage. - If using traditional wire guide port, cover wire control ports, introduce tip of device into accessory channel and advance in short increments until tip is endoscopically visible.
Then Refer to Steps 2-5 Below
- Following cannulation, contrast may be injected through injection port to fluoroscopically confirm position of device. (See Fig. 2)
- Following electrosurgical unit manufacturers instructions, verify desired settings and proceed with sphincterotomy.
- Upon completion of sphincterotomy, turn surgical unit off.
Note: Previously placed wire guide may be left in position, in order to facilitate introduction of compatible devices (if applicable). - Disconnect active cord from device handle and from electrosurgical unit. Wipe active cord with a damp cloth to remove all foreign matter. Store in a loose coil.
Note: Wrapping active cord tightly may damage device.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
