Description
Cook Medical G22674 - BRUSH, CYTOMAX II, DBL LUMEN, CYTOLOGY, EACH
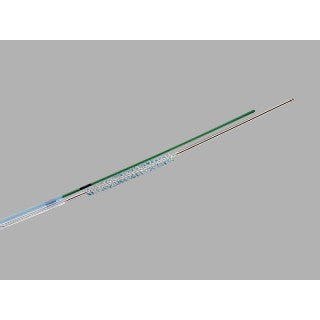
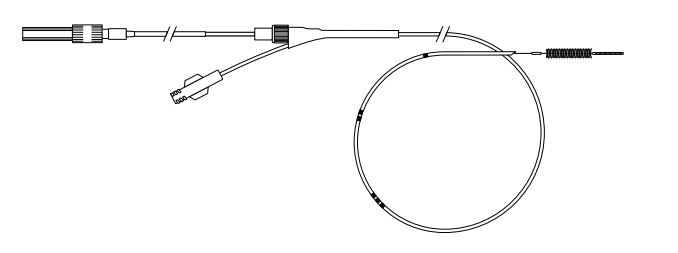
Cytomax II Double Lumen Cytology Brush
| Order Number | Reference Part Number | Catheter Size Fr | Catheter Length (cm) | Wire Guide Diameter inch | Brush Diameter (mm) | Brush Length (cm) | Filiform Tip Size (cm) | Minimum Accessory Channel (mm) |
| G22674 | DLB-35-1.5-S | 8 | 200 | 0.035 | 3 | 2.5 | 1.5 | 3.2 |
Intended Use
This device is used to collect cells in the biliary system.
NOTES:Do not use this device for any purpose other than stated intended use. If package is opened or damaged when received, do not use. Visually inspect with particular attention to kinks, bends and breaks. If an abnormality is detected that would prohibit proper working condition, do not use.
Please notify Cook for return authorization. Store in a dry location, away from temperature extremes. Use of this device restricted to a trained healthcare professional.
Contraindications
Those specific to ERCP and any procedure to be performed in conjunction with cell collection.
Potential Complications
Those associated with ERCP include, but are not limited to: pancreatitis, cholangitis, aspiration, perforation, hemorrhage, infection, sepsis, allergic reaction to contrast or medication, hypotension, respiratory depression or arrest, cardiac arrhythmia or arrest.
Precautions
Refer to package label for minimum channel size required for this device.
Instructions for Use
- Uncoil device, remove protective tubing from brush.
- Retract brush completely into sheath.
Note: Do not retract end of filiform tip of brush beyond radiopaque band on tip of catheter.
Note: Prior to advancing device over a pre-positioned wire guide, flush wire guide lumen with sterile water or saline to facilitate advancement. - Introduce device into accessory channel of endoscope and advance in short increments until device is endoscopically visualized exiting scope.
- Following cannulation, advance device under fluoroscopic monitoring to desired position. Contrast may be injected while wire guide is in place using a side arm adapter placed over wire guide and attached to Luer lock fitting on device.
- Advance brush out of sheath, then brush area to be sampled to obtain adequate cellular material.
Note: Endoscope elevator should be open prior to brushing to allow for easier movement. - After obtaining specimen, retract brush into sheath. When removing device from endoscope, wire guide may be left in place to facilitate introduction of other compatible wire guided devices. If wire guide was removed during brushing, maintain ductal access by reintroducing wire guide into duct prior to removing device.
- Prepare specimen per institutional guidelines.