Description
Cook Medical G23707 - SPECTRUM, SINGLE LUMEN, TURBO-JET, MINO / RIF, EACH
Cook Spectrum Turbo-Ject PICC Set - Minocycline/Rifampin Impregnated
Cook Spectrum Turbo-Ject Peripherally Inserted Central Venous Catheters feature a soft polyurethane suture wing for secure fixation to the skin. Reinforced polyurethane extensions reduce catheter damage during catheter manipulations. Catheters can be trimmed to fit the patients anatomy. Plastic clamp(s) help prevent air aspiration due to inadvertent hub dislodgment.
Cook Spectrum Turbo-Ject Peripherally Inserted Central Venous Catheters are impregnated with the antimicrobial agents minocycline and rifampin. The highest average concentrations (i.e., for the 6 Fr. triple lumen catheter) are 499 g/cm and 561 g/cm respectively. The activity of the antimicrobial agents minocycline and rifampin is localized at the internal and external catheter surface, and is not intended for treatment of existing infection. The antimicrobial agents, minocycline and rifampin, have a yellow and an orange appearance; therefore, some coloration of the catheter is normal.
| Single Lumen Catheter | ||||||
| Item Number | Catheter | Outer Diameter | Inner Diameter | |||
| G23707 | French | Inch | Gage | Inch | Gage | Lumen Vol. (mL) |
| 3 | 0.041 | 19 | 0.027 | 19 | 0.52 | |
Set Components
- Cook Spectrum Turbo-Ject Power-Injectable PICC
- 21 gage, 7 cm long EchoTip percutaneous entry needle*
- Wire guide
- Locking Peel-Away introducer sheath**
- 12 mL disposable syringe
- 130 cm or 150 cm long marked wire in radiology (OTW) sets only
- Hydrophilic-coated obturator in standard and bedside sets only
- Disposable safety scalpel
- Paper measuring tape
- Needleless injection caps (one per lumen)
- Catheter securement device
- Catheter ID card
- Safety sharps container
Features & Benefits
- The orange color indicates that the PICC has Spectrum technology that helps protect against CLABSIs.
- Depth markings every 1 cm help you insert the PICC accurately.
- The extension tube label identifies the maximum power injection (CT) flow rates.
- Low-profile clamps
- Clearly marked hubs:
- French size
- Gage
- Maximum CT flow rates
- Lumen volume
- Reverse-taper shaft design
Intended Use
Cook Spectrum Turbo-Ject Peripherally Inserted Central Venous Catheter (PICC) Sets are intended for short- or long-term use for venous pressure monitoring, blood sampling, administration of drugs and fluids, and for use with power injectors for delivery of contrast in CT studies. The catheter is impregnated with the antimicrobials minocycline and rifampin to help provide protection against catheter-related bloodstream infections (CRBSIs). The Cook Spectrum Turbo-Ject PICC is indicated for multiple injections of contrast media through a power injector. The maximum pressure limit setting for Power Injectors used with the Spectrum Turbo-Ject PICC may not exceed 325 psi and the flow rate may not exceed the maximum flow rate indicated, as shown on the following table.
| Catheter Size | Maximum Flow Rate* | Injection Pressure Limit Setting |
| 3 Fr Single Lumen | 2 mL/sec | 325 psi |
*Flow rates achieved using room temperature Omnipaque 300 contrast and verified using a MEDRAD Stellant CT injector system. Omnipaque 300 has a viscosity of 11.8 centipoise at room temperature (20 degrees C). A change in temperature or viscosity of the contrast medium used will result in a change in achievable flow rates.
Cook Spectrum Turbo-Ject Power Injectable PICC Dynamic and Static Pressure Results
| French/Lumen |
Priming Volume (mL) |
Maximum Labeled Flow Rate (mL/sec) |
Average Maximum Catheter Pressure During Maximum Flow (psi) |
Average Static Burst Pressure in 37? Water (psi) |
Range of Static Burst Pressure in 37? Water (psi)** |
| 3.0/Single | 0.52 | 2 | 123 | 249 | 237-258 |
Catheters Used in the Clinical Study
| Type of Catheter | Control Cohort | Treatment Cohort |
| Double-Lumen Subclavian | 84 (48%) | 84 (46%) |
| Single-Lumen Subclavian | 24 (14%) | 34 (19%) |
| PICC Line | 66 (38%) | 64 (35%) |
| Total | 174 (100%) | 182 (100%) |
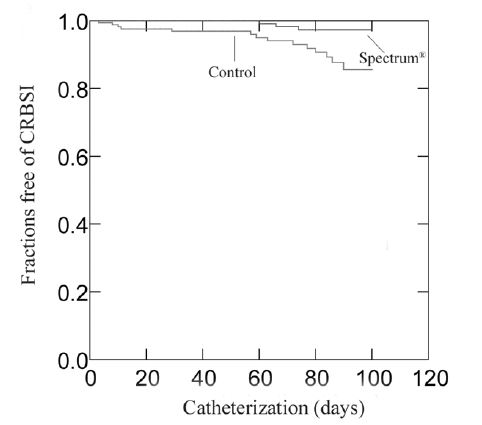
Results from the clinical study showed a statistically significant decrease in the incidence of catheter-related bloodstream infection in patients receiving the Cook Spectrum Silicone Catheter, with infections occurring in 3 of 182 patients (2%) as compared to 14 of 174 patients (8.0%) for the control catheter (p = .005).
Organisms isolated from patients having catheter-related bloodstream infection in the treatment cohort included Candida parapsilosis and Klebsiella pneumonia. Testing of isolates revealed no evidence of resistance to minocycline or rifampin developed.
Blood samples obtained from 9 patients at 1-2 days after insertion of the Cook Spectrum Silicone Catheter were assayed for minocycline and rifampin by high-performance liquid chromatography (HPLC) analysis. No detectable systemic levels of minocycline or rifampin were observed (limit of detection = 1.0 g/mL for both antimicrobials).
The rates of catheter-related bloodstream infection (calculated according to CDC definition) were 0.24 per 1,000 catheter-days for treatment catheters and 1.30 per 1,000 catheter-days for control catheters. Kaplan-Meier survival analysis indicated that Cook Spectrum Silicone Catheters were, over time, associated with a significantly lower risk of catheter-related bloodstream infection than the control catheters (p = 0.003 by log-rank test).
Kaplan-Meier Survival Curves for Freedom from Catheter-Related Bloodstream Infection (CRBSI)
Discussion of Antimicrobial Activity
Antimicrobial activity associated with the Cook Spectrum Silicone Central Venous Catheter over time has been demonstrated in the following way: The length of activity of the antibiotics was established during in vitro zone of inhibition testing after suspension in saline at 37 degrees C. Antimicrobial activity in the 7.0 French double-lumen catheter was demonstrated for at least 28 days against Staphylococcus epidermidis, the most common organism implicated in catheter-related infection.
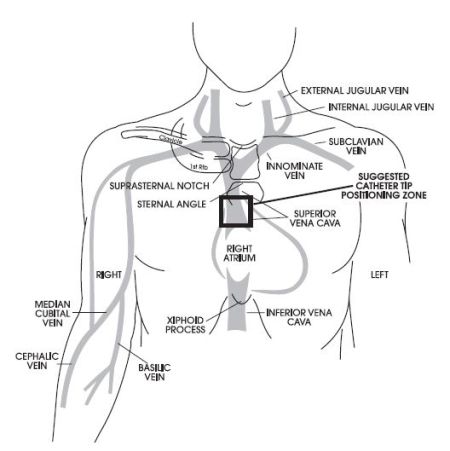
Catheter Tip Positioning
How Supplied
Supplied sterilized by ethylene oxide gas in peel-open packages. Intended for one-time use. Sterile if package is unopened or undamaged. Do not use the product if there is doubt as to whether the product is sterile. Store in a dark, dry, cool place. Avoid extended exposure to light. Upon removal from package, inspect the product to ensure no damage has occurred.

