Description
Cook Medical G24237 - BALLOON, POSTPARTUM, BAKRI, J-SOSR-100500, EACH
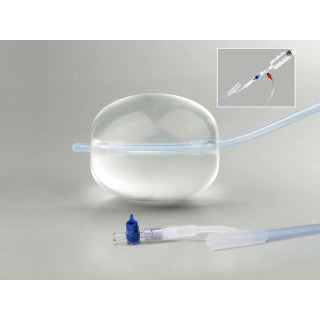
Bakri Postpartum Balloon with Rapid Instillation Components
The Bakri Postpartum Balloon is a silicone balloon catheter with a maximum inflation volume of 500 mL. The Rapid Instillation Components include polymer tubing with an IV bag spike and three-way valve.This device is intended to provide temporary control or reduction of postpartum uterine bleeding when conservative management is warranted. The set includes a 60 mL syringe, extension tubing with bag spike, and dual check valve. Please refer to the Instructions for Use for complete information on product usage, indications, and contraindications.
| Order Number | Reference Part Number | Catheter Fr | Length (cm) | Balloon Volume mL |
| G24237 | J-SOSR-100500 | 24 | 54 | 500 |
Features and Benefits
- Can be used following vaginal or cesarean delivery
- Includes rapid instillation components to facilitate inflation of the balloon
- May be used with B-Lynch compression sutures if clinically warranted
- Is constructed of latex-free silicone
Sequential Procedure Considerations for Conservative Management of Uterotonic-Resistant PPH
| Vaginal birth | Cesarean birth |
| 1. Bimanual massage | 1. Fundal massage |
| 2. Repair of any lacerations | 2. Inspection of the broad ligament and posterior uterus and inspection for the presence of retained placenta |
| 3. Dilation and curettage to rule out retained placenta | 3. B-Lynch suture |
| 4. Placement of intrauterine balloon | 4. Placement of intrauterine balloon |
| 5. Selective embolization | 5. Uterine artery ligation |
Intended Use
This device is intended to provide temporary control or reduction of postpartum uterine bleeding when conservative management is warranted.
Contraindications
- Arterial bleeding requiring surgical exploration or angiographic embolization
- Cases indicating hysterectomy
- Pregnancy
- Cervical cancer
- Purulent infections in the vagina, cervix, or uterus
- Untreated uterine anomaly
- Disseminated intravascular coagulation
- A surgical site that would prohibit the device from effectively controlling bleeding
Precautions
- This product is intended for use by physicians trained and experienced in obstetrics and gynecological techniques.
- Avoid excessive force when inserting the balloon into the uterus.
Instructions for Use
Important: Prior to transvaginal or transabdominal placement of the Bakri Postpartum Balloon, the uterus should be free of all placental fragments, and the patient should be evaluated to ensure that there are no lacerations or trauma to the genital tract and that the source of the bleeding is not arterial.
Transvaginal Placement
- Determine uterine volume by direct examination or ultrasound examination.
- Insert the balloon portion of the catheter into the uterus, making certain that the entire balloon is inserted past the cervical canal and internal ostium.
- Place an indwelling urinary bladder Foley catheter at this time, if not already in place, to collect and monitor urine output.
Transabdominal Placement, Post-Cesarean Section
- Determine uterine volume by direct examination.
- From above, via access of the cesarean incision, pass the tamponade balloon, inflation port first, through the uterus and cervix.
NOTE: Remove the stopcock to aid in placement and reattach prior to filling balloon. - Have an assistant pull the shaft of the balloon through the vaginal canal until the deflated balloon base comes into contact with the internal cervical ostium.
- Close the incision per normal procedure, taking care to avoid puncturing the balloon while suturing.
NOTE: Ensure that all product components are intact and the hysterotomy is securely sutured prior to inflating the balloon. If clinically relevant, the abdomen may remain open upon inflation of the balloon to closely monitor uterine distention and confirm the hysterotomy closure.
NOTE: If clinically relevant, a B-Lynch compression suture may be used in conjunction with the Bakri Postpartum Balloon.
Balloon Inflation With Syringe
WARNING: Always inflate the balloon with sterile liquid. Never inflate with air, carbon dioxide or any other gas.
WARNING: The maximum inflation is 500 mL. Do not overinflate the balloon. Overinflation of the balloon may result in the balloon being displaced into the vagina.
NOTE: To ensure that the balloon is filled to the desired volume, it is recommended that the predetermined volume of fluid be placed in a separate container, rather than relying on a syringe count to verify the amount of fluid that has been instilled into the balloon.
- Place an indwelling urinary bladder Foley catheter at this time, if not already in place, to collect and monitor urine output.
- Using the enclosed syringe, begin filling the balloon to the predetermined volume through the stopcock.
- Once the balloon has been inflated to the predetermined volume, confirm placement via ultrasound.
NOTE: See Fig. 1 for proper placement.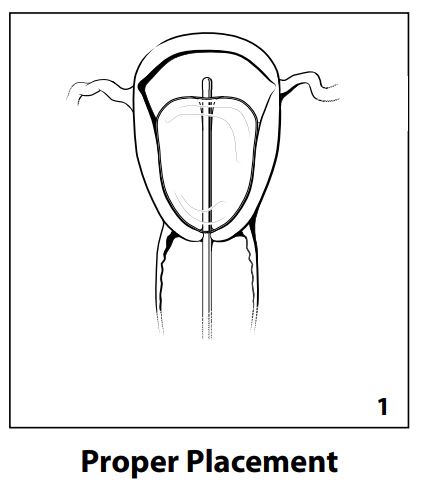
- If desired, traction can be applied to the balloon shaft. In order to maintain tension, secure the balloon shaft to the patients leg or attach to a weight, not to exceed 500 grams.
NOTE: To prevent displacement of the balloon into the vagina, counterpressure can be applied by packing the vaginal canal with iodine- or antibiotic-soaked vaginal gauze. - Connect the drainage port to a fluid collection bag to monitor hemostasis.
NOTE: To adequately monitor hemostasis, the balloon drainage port and tubing may be flushed clear of clots with sterile isotonic saline. - Monitor the patient continuously for signs of increased bleeding and uterine cramping.
Balloon Inflation With Rapid Instillation Components
- See Figs. 2-8, at the front of this booklet.


NOTE: Ultrasound should be used to confirm proper placement of the balloon once the balloon is inflated to the predetermined volume.
Balloon Removal
NOTE: The timing of balloon removal should be determined by the attending clinician upon evaluation of the patient once bleeding has been controlled and the patient has been stabilized. The balloon may be removed sooner upon the clinicians determination of hemostasis. The maximum indwell time is 24 hours.
- Remove tension from the balloon shaft.
- Remove any vaginal packing.
- Using an appropriate syringe, aspirate the contents of the balloon until fully deflated. The fluid may be removed incrementally to allow periodic observation of the patient.
NOTE: In an emergent situation, the catheter shaft may be cut to facilitate more rapid deflation. - Gently retract the balloon from the uterus and vaginal canal and discard.
- Monitor patient for signs of bleeding.
How Supplied
Supplied sterilized by ethylene oxide gas in peel-open packages. Intended for one-time use. Sterile if package is unopened and undamaged. Do not use the product if there is doubt as to whether the product is sterile. Store in a dark, dry, cool place. Avoid extended exposure to light. Upon removal from the package, inspect the product to ensure no damage has occurred.
CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician (or a properly licensed practitioner).