Description
Cook Medical G49804 - SPECTRUM, ANTI-IMPREGNAT TRIPLE LUM POLY, EACH
Cook Spectrum Central Venous Catheter Trays (Maximal Sterile Barrier Trays - Polyurethane), Cap, Mask and Gown Included
Cook polyurethane central venous catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Sets and trays also contain an appropriately sized access needle, a wire guide and accessories for use in percutaneous vascular access procedures. The device and its components are not made with natural rubber latex.
The Spectrum procedural tray has been uniquely designed to support your hospitals process bundle. It contains several components that comply with national recommendations for patient safety from AHRQ, CDC, IHI, Joint Commission, OSHA and SHEA Compendium.
7.0 Fr Triple Lumen
Curved Needle with Suture and Holder
| Order Number | Reference Part Number | Catheter (Fr) | Equivalent gage |
Catheter Length (cm) |
Wire Guide Diameter (inch) |
Fits Needle gage |
| G49803 | C-UTLMY-701J-ABRM-HC-IHI-FST-A-RD | 7.0 | 13 | 15 | 0.032 | 18 TW |
Tray Contains

*Available only on 7, 8, 9 and 10 Fr catheters.
**Selected catheter lengths only.
Intended Use
Cook Spectrum central venous catheters are intended for:
- Continuous or intermittent drug infusions
- Central venous blood pressure monitoring (CVP)
- Acute hyperalimentation
- Blood sampling
- Delivery of whole blood or blood products
- Power injection of contrast media*
The activity of the antimicrobial agents, minocycline and rifampin, is localized at the internal and external catheter surface and helps to provide protection against catheter-related bloodstream infections (CRBSI). It is not intended for treatment of existing infection. The device is a short-term use catheter.
Technical Features
| Catheter Size1 | Maximum Power Injection Flow Rate (mL/sec)2 | Average Maximum Internal Catheter Pressure During Maximum Flow Rate (psi)3 | Average Maximum Dynamic Burst Pressure (psi) | Average Maximum Static Burst Pressure (psi)4 |
| 7 French, Triple Lumen | 10 | 143.3 | 288.7 | 181.6 |
- 1All testing performed on the distal lumen of each catheter.
- 2Pressurized flow rates are determined with injector safety cut-off set at 325 psi and contrast agent at room temperature.
- 3Pressures determined using room temperature Omnipaque 300 contrast and verified using a Medrad Stellant CT injector system with injector safety cut-off at 325 psi. Omnipaque 300 has a viscosity of 11.8 centipoise at 20 degrees C. A change in temperature or viscosity of the contrast medium used will result in a change in achievable flow rates. Omnipaque 300 is a registered trademark of GE Healthcare.
- 4Maximum Static/Burst pressure is the static burst pressure failure point of the catheter. When catheter was occluded, failure occurred at these pressures.
Triple-Lumen Information
| French Size | Lumens | Equivalent Gage | Minimum Lumen Volume |
| 7.0 | #1 #2 #3 |
16 18 18 |
0.6 mL 0.5 mL 0.6 mL |
Suggested Lumen Utilization: Triple-Lumen
- #1 Distal exit port (endhole) -- whole blood or blood product delivery and sampling; any situation requiring more flow rate; CVP monitoring; medication delivery; power injection studies. It is strongly recommended that this lumen be used for all blood sampling. CT labeled on the distal #1 lumen hub indicates that this is the lumen which should be utilized for power injection.
- #2 Middle exit port -- medication delivery; acute hyperalimentation.
- #3 Proximal exit port -- medication delivery.
Proven Lowest Infection Rates
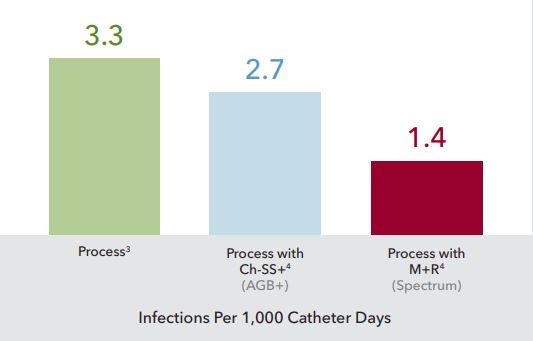
More than two decades of evidence, including over 21 peer-reviewed studies and meta-analyses, prove Spectrums ability to help prevent catheterrelated bloodstream infections (CRBSIs), without causing bacterial resistance. Thats evidence of protection no other process or technology can match. As an additional benefit, Spectrum CVCs are available in comprehensive procedural trays with all the necessary components for successful and efficient bedside insertion.
First Trial of Second Generation AGB+ and Spectrum
Proven Cost Savings
The cost to treat just one line infection can range from $11,9715 to $56,000. And with 250,000 cases of CRBSI reported in the U.S. each year,6 these infections can create a significant economic burden for hospitals. In a challenging clinical environment, a hospital that switches to Spectrum may expect to see a decrease in CRBSI rates, attributable mortality and CRBSI-related costs. What might not be so apparent is that even high-performing hospitals can switch to Spectrum to drive incremental improvement in CRBSI rates and still achieve substantial reductions in mortality and cost.