Description
Product Description
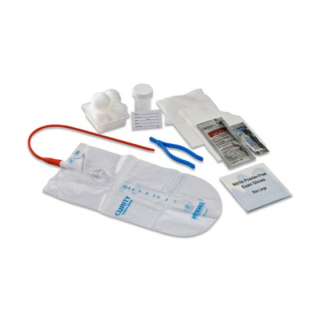
Covidien 2480 - Dover Closed Intermittent Tray with Vinyl Catheter, BZK Swabs, 14 Fr, 20/CS
Covidien 2480 Dover Closed Intermittent Tray with Vinyl Catheter
Dover closed intermittent trays include (unless indicated otherwise) a CSR wrap, an underpad, blue nitrile exam gloves, antiseptic, lubricating jelly, a specimen container, and 1500 mL collection bag.
Device Characteristics of Covidien 2480 Dover Closed Intermittent Tray with Vinyl Catheter
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): |
Yes |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | Yes |
| Combination Product: | Yes |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |