Description
Product Description
Edwards Lifesciences 120803F - Fogarty Arterial Embolectomy Catheter, 80cm, 3Fr, Each
Edwards Lifesciences 120803F Fogarty Arterial Embolectomy Catheter
Removal of fresh, soft emboli and thrombi from vessels in the arteries of the non-central circulatory system
- Hand-tied using a recessed winding technique to secure balloon to catheter
- Balloon exhibits a symmetry that exerts uniform contact with vessel walls for even pressure and
- precise traction
- Rounded tip promotes easy insertion for reduced trauma
Edwards Lifesciences 120803F Fogarty Arterial Embolectomy CatheterChart
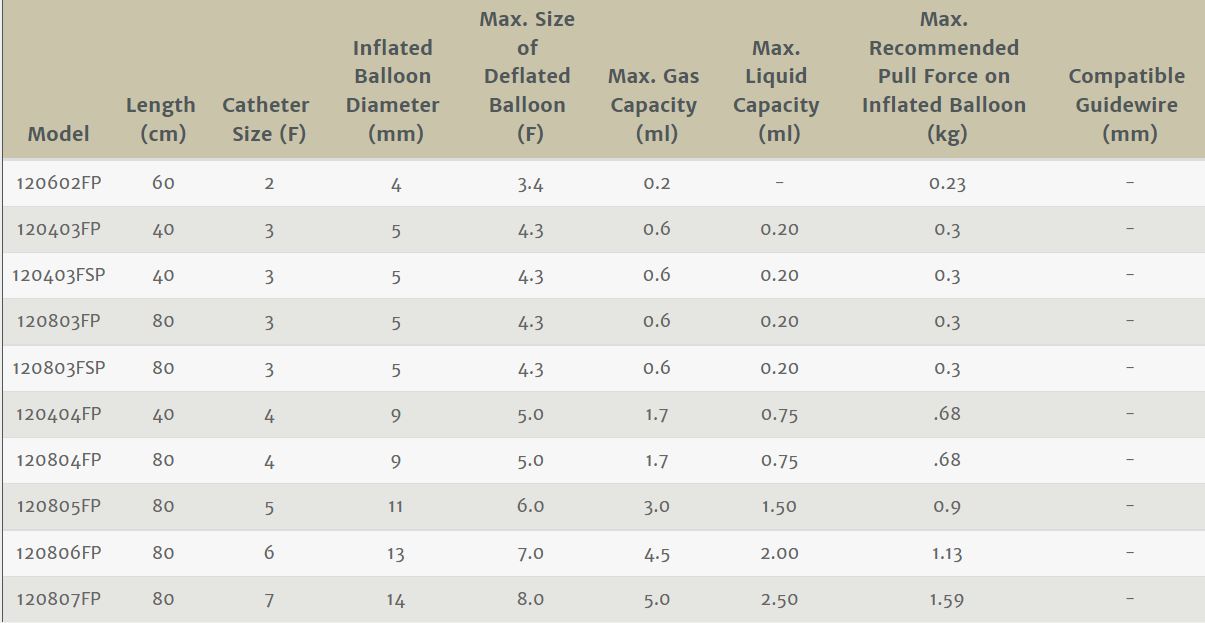
Edwards Lifesciences 120803F Fogarty Irrigation Catheter Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | Yes |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | Yes |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
