Description
Product Description
Edwards Lifesciences 12TLW403F - Over-the-wire Fogarty Thru-Lumen Embolectomy Catheters, 40CM, 3 FR, Each
Edwards Lifesciences 12TLW403F Over-the-wire Fogarty Thru-Lumen Embolectomy Catheters
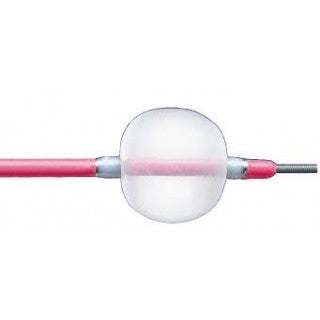
Multi-use guidewire-compatible design enables removal of fresh, soft emboli and thrombi from vessels in the arterial system as well as temporary occlusion of blood vessels, infusion of fluids, and blood sampling.
- Tough, compliant Fogarty balloon conforms to the vessel to maximize clot removal while minimizing trauma
- Fogarty balloon conforms to the vessel to maximize clot removal while minimizing trauma
- Provides the solution for distal thrombus removal in the arterial system
- Stainless steel bushings prox/distal of the balloon are very radiopaque
- Also, helps provide solution for distal thrombus removal in arterial system
- Radiopaque stainless-steel bushings
- Barium addition on catheter shaft for radiopacity
Edwards Lifesciences Over-the-wire Fogarty Thru-Lumen Embolectomy Catheters Chart
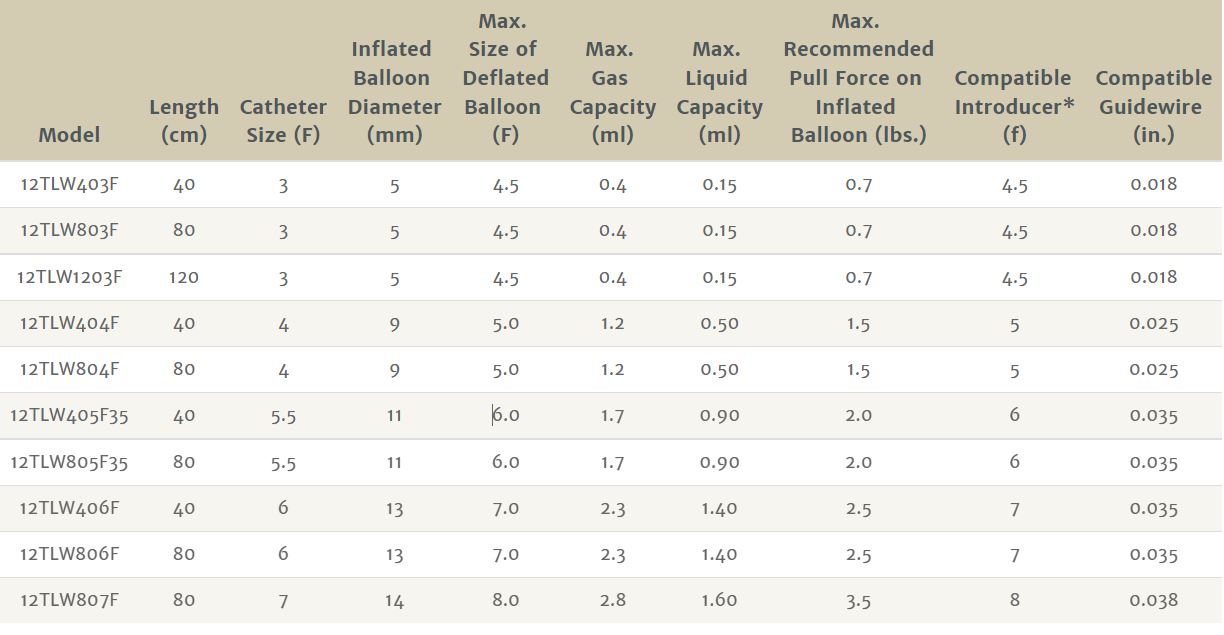
Edwards Lifesciences 12TLW403F Fogarty Irrigation Catheter Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | Yes |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | Yes |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |