Description
Product Description
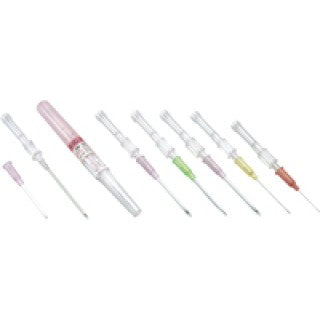
Exel International 26735 - SAFELET IV Catheter (Pen Type), 18G x 1 1/4", Green, EACH
Exel International 26735 SAFELET IV Catheter (Pen Type)
Exel IV Catheter is designed to prevent roll-back for smooth, comfortable insertion. Color-coded for easy size identification. Available in a wide range of sizes.
- Sterile, Non-Toxic, Non-Pyrogenic
- Functional design causing permitting one action opening and no rolling
- Color-coded casing cap allows for easier identification of catheter size
- Translucent catheter hub and flashback chamber allows for easy detection of blood flashback at vein insertion
- Precision finished FEP catheter assures stable flow and eliminates catheter tip kink during venipuncture
- Easy dispenser pack
- Radio opaque catheter
- Cannula with additional back bevel
- Can be connected to syringe by removing filter cap to expose Luer taper end
- Use of hydrophobic membrane filter eliminates blood leakage
- Close and smooth contact between catheter tip and inner needle enables safe and smooth venipuncture
- Crimped smooth engagement between catheter and hub, without use of adhesive, helps eliminate blood residuals and helps reduce blood coagulation or hemolysis
- This product does not contain Natural Rubber Latex
Exel International 26735 SAFELET IV Catheter (Pen Type) Specifications
| Catalog No. | Size | Gauge |
| 26735 | 18G x 1 1/4" | 18G |
| Outside Diameter (mm) | Total Length (mm) | Hub Color |
| 0.9 mm | 47.0 mm | Green |
Device Characteristics of Exel International 26735 SAFELET IV Catheter (Pen Type)
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |