Description
Product Description
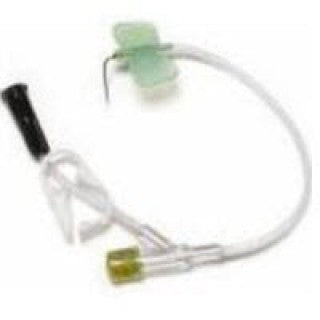
MDC Research LH-0029YN - Huber Needle Safety 2Clamps & LL 22Gx3/4" 25/CS
SafeStep Huber Needle Set with Y-Injection Site and Medegen Needleless Injection Cap, 22 Gauge x .75"
The SafeStep Huber Needle Set is a device intended for insertion into the septum of subcutaneously implanted ports and infusion of fluids into the port. The SafeStep* Huber Needle safety feature is manually activated during needle removal, and is designed to aid in the prevention of accidental needle sticks.
- Rotating needle head for maneuvering and positioning
- Small stable design ideal for patient comfort and easy to dress
- Wingless design
- Low Profile (0.550")
- Padded footprint for comfort
- Dual lumen port adaptable
Warnings
- Fully tighten all connectors, Y-site end caps, or needleless connectors before use. Failure to attach an end cap after removing a male Luer locking end cap can result in an air embolism or bleeding.
- This is a single-use product. DO NOT REUSE. DO NOT RESTERILIZE.
- Failure to use this device correctly when removing needle from port site could result in the needle tip re-emerging from the base, resulting in an accidental needlestick with a contaminated needle. A needlestick with a contaminated needle may cause infectious disease.
- Verify needle length is correct based on port reservoir depth, tissue thickness, and the thickness of any dressing beneath the bend of the needle; if too long, needle and/or port may be damaged at insertion; if too short, needle may not completely pierce port septum, and medication may be delivered into surrounding tissue and/or needle may be blocked.
- Handle and discard in accordance with accepted medical practice and all applicable regulations. After use, this product is a potential biohazard.
- Do not alter this device.
- Needless Y-injection site valve may leak slightly at certain pressures resulting in a small bead of fluid on the valve.
- The textured handle and base are not connected. The base will move along the length of the needle.
Cautions
- Carefully read and follow all instructions prior to use.
- Care must be taken to avoid accidental needlesticks.
- Only qualified healthcare practitioners should insert, manipulate and remove these devices.
- High pressure or use with power injectors may cause leakage or damage.
- Confirm correct needle placement in the port reservoir by aspiration of blood before infusion of any substance. If there is doubt regarding proper needle placement, preform a radiographic dye procedure to confirm placement per institutional protocol.
- Do not remove and reinsert the needle into the port.
- Avoid excessive manipulation once the needle is in the port.
- Follow all instructions, contraindications, warnings, cautions and precautions for all infusates, ports, IV pumps, IV sets and needleless systems, as specified by the respective manufacturer.
- Follow standard infection control precautions as specified by the CDC (USA) or local equivalent.
- Do not use this product if it appears damaged or if the package has been previously opened or damaged.
- Do not access the needleless Y-injection site valve with a needle. Valve puncture may result in an embolism.