Description
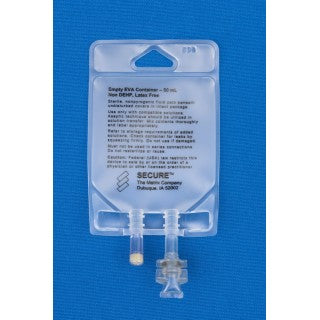
Metrix-Secure 66042 - 50ml EVA 2-Port Bag, 50/CS
50 ml Ethyl Vinyl Acetate (EVA) 2 Port Bag
Ethyl Vinyl Acetate (EVA) bags are constructed from a copolymer, monolayer film
Our EVA single-use bags are designed for parenteral applications. They are made from high quality, Class VI film
- EVA container.
- Non-DEHP bag, ports and tubing.
- Latex free.
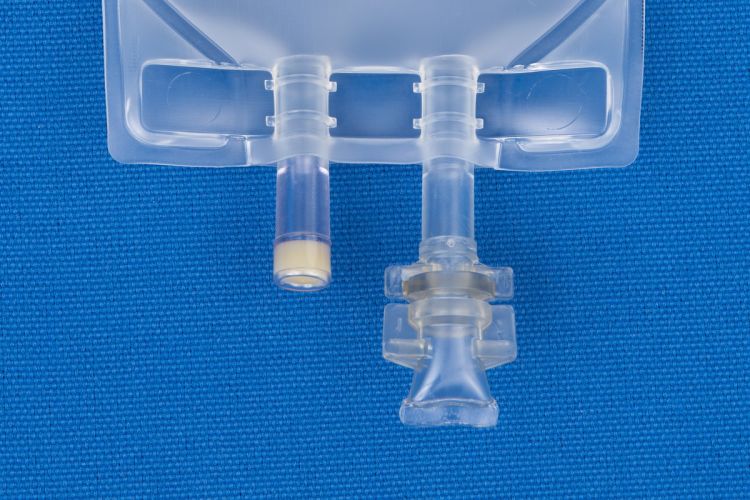
- 2 Ports.
- (i) Injection Site
- (ii) Patient access spike port.
- For filling through injection site.
These bags are specifically designed to provide strong seals, extraordinary robustness, superior flex, crack and pin-hole resistance
Storing
EVA must be stored at temperature under 40 degree celsius. The sun radiation, heat, humidity is prohibit.
DEHP
Infusion Therapy Standards of Practice advise to use administration sets not made with di-ethylhexyl-phthalate (DEHP) to administer lipid-based infusates, such as IVFE or TNA. DEHP is lipophilic and is extracted into the lipid solution with commonly used polyvinyl chloride administration sets and containers. DEHP is considered a toxin, and studies have demonstrated increased DEHP levels in lipid solutions, which is especially a risk with neonatal, pediatric, and long-term home care patients (42).