Description
Metrix-Secure 66430 - 3000ml EVA Legless Bag Male Screw Connector, 50 Per/Cs
3000 ml Ethylene Vinyl Acetate (EVA) Legless Bag with Male Screw Connector
Ethyl Vinyl Acetate (EVA) bags are built from a single-web, medical grade laminate of four different films, with a fluid contact surface of EVA, with two layers of low-density polyethylene (LDPE) enveloping a layer of ethyl vinyl alcohol (EVOH).
EVA bags are high-quality bags made for laboratory, diagnostic as well as biopharmaceutical applications. These bags provide exceptional seals and robustness, good flexibility, resistance to cracks and pinholes and a desirable gas and moisture barrier performance.
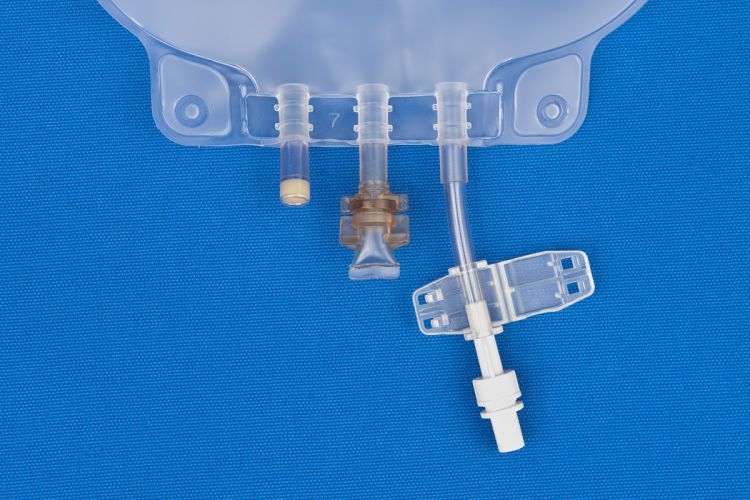
3000 ml Ethylene Vinyl Acetate (EVA) Characteristics:
- EVA container
- Non-DEHP bags, ports and tubing
- Latex Free
- Fill leg with male screw connector and female threads
- Extremely flexible by providing the fill legs separately
- Fill legs available for most TPN compounding and gravity filling applications
- EVA legless bags come with an attached non-reopening clamp
Ethylene Vinyl Acetate Benefits
EVA is a elastomeric polymer that generates materials that resemble rubber in their softness and flexibility. This material provides a glossy finish with good clarity, low-temperature resistance, hot-melt adhesive waterproof properties, as well as resistance to UV radiation. EVA has a scent similar to vinegar and is the competitive material with rubber and vinyl products in various electrical applications.
EVA must be kept at a temperature under 40 degree celsius.
Non-DEHP
IV Therapy Practice Standards advise the use of non-DEHP administration sets when administering lipid-based infusates, such as IVFE or TNA. DEHP is a toxin, studies have shown that the component causes toxic effects on reproduction and fetal development.