Description
Product Description
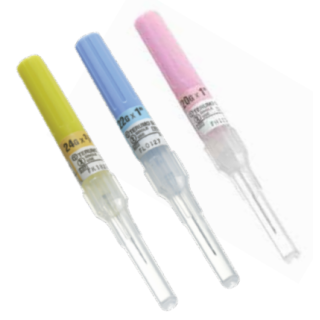
Terumo SR-OX2025CA - SURFLO ETFE I.V. Catheter, 20G x 1", 200/CS
Terumo SR-OX2025CA SURFLO ETFE I.V. Catheter
A sharp double-bevel introducer needle plus a medical grade lubricant allow for easier penetration and smoother travel through tissue. Preview chamber in clear sure-grip hub gives immediate indication of proper placement. Flexible thinwall catheter with large inner diameter assures good blood flow. All this plus easy-to-handle, tamper-evident packaging make SURFLO I.V. Catheters a great clinical value.
Device Characteristics of Terumo SR-OX2025CA SURFLO ETFE I.V. Catheter
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |